Introduction
Styrene monomer is used to produce polystyrene, which has a wide range of applications, the most common being in packaging and insulation, such as polystyrene foam cups. Styrene is produced by dehydrogenating ethylbenzene. Ethylbenzene is formed from the reaction of ethylene and benzene, and one way to produce benzene is by hydroalkylation or transalkylation of toluene, which is obtained as a byproduct of gasoline production. Very little ethylbenzene is sold commercially. Most ethylbenzene producers convert it directly to styrene in the same plant.
Process Description
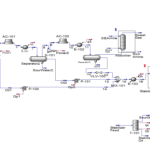
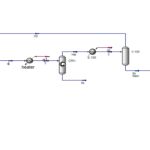
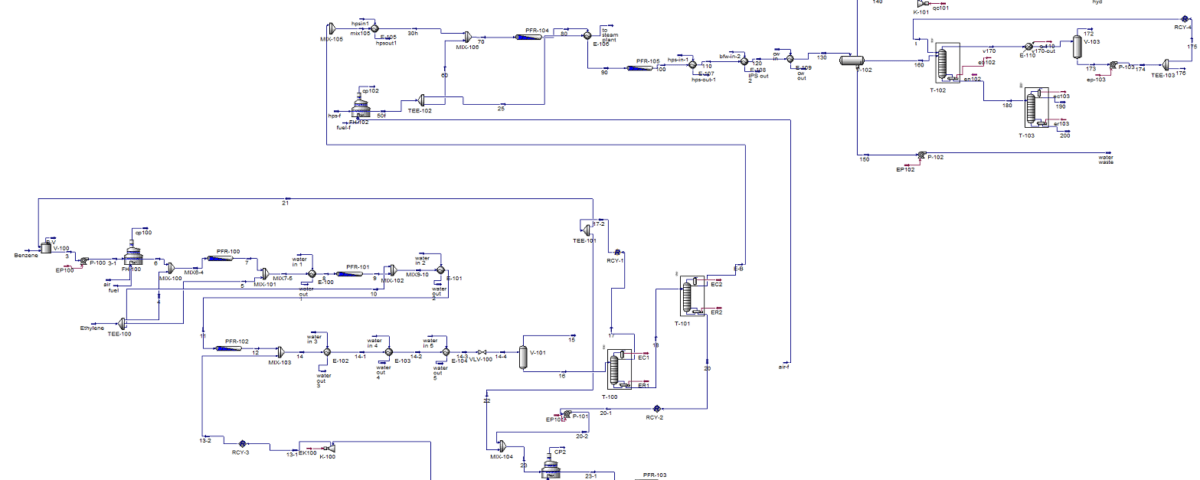
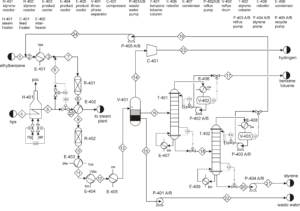
Ethylbenzene, along with superheated steam, is fed into fixed bed reactors where it is converted to styrene. This reaction is endothermic, and the presence of steam provides the necessary energy while shifting the reaction equilibrium towards product formation. After cooling the reactor effluent, the gaseous, organic liquid, and aqueous products are separated. The recovered hydrogen and benzene and toluene impurities are either burned or recycled to the ethylbenzene production unit. Pure styrene is separated from ethylbenzene by distillation. To prevent spontaneous polymerization of styrene, the entire process is carried out at low pressure and controlled temperature.
Conclusion
The production of styrene from benzene is a pivotal industrial chemical process that serves as the foundation for a vast array of polymeric products. This process typically involves two primary stages: the alkylation of benzene with ethylene, followed by the dehydrogenation of the resulting ethylbenzene. The significance of styrene as an aromatic monomer in various industries, especially in the production of polystyrene, unsaturated polyester resins, and synthetic rubbers, is undeniable. However, this process encounters challenges such as selecting the appropriate catalyst, precisely controlling reaction conditions, and effectively separating products.
Simulation of Styrene Production Process from Benzene
In this project, the styrene production process from benzene has been simulated using Aspen HYSYS version 14. A comprehensive report accompanies the project.