Introduction
Isopropyl alcohol is a colorless, flammable liquid with a pungent odor. It is widely used as a solvent and disinfectant, particularly for dissolving oils. Isopropanol evaporates rapidly due to its relatively low boiling point. It is readily soluble in water, benzene, and most organic solvents but is insoluble in salt solutions. It is also known in the chemical market as isopropanol, 2-propanol, or dimethyl carbinol.
Production Methods of Isopropyl Alcohol
There are two primary methods for producing isopropyl alcohol, both of which utilize propene as a feedstock:
- Direct hydration of propene using a homogeneous acid catalyst
- Indirect hydration of propene using a heterogeneous acid catalyst
In the indirect method, propene first reacts with sulfuric acid, and subsequently, the produced ester is hydrolyzed to form isopropyl alcohol. In direct hydration, propene and water react in the presence of a tungsten catalyst. The direct hydration method is more commonly used in industry as it avoids corrosion issues associated with acids. However, the direct hydration method cannot achieve high purity isopropyl alcohol from propene.
A novel method for producing isopropyl alcohol has been proposed. In this method, which utilizes a reactive distillation column, a transesterification reaction occurs between isopropyl acetate and methanol. The reaction conversion is approximately 99%, and high-purity isopropyl alcohol can be produced through distillation. [5]
This project focuses on simulating the production process of isopropyl alcohol from isopropyl acetate using a reactive distillation column as proposed by Yufeng and colleagues in 2019. The following figure shows the flowsheet of this process.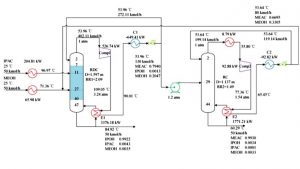 This project involves the simulation of isopropyl alcohol production in a reactive distillation column using Aspen Plus software. The project comes complete with a training video and a comprehensive report.
This project involves the simulation of isopropyl alcohol production in a reactive distillation column using Aspen Plus software. The project comes complete with a training video and a comprehensive report.