Introduction
Ethylene oxide, also known as oxirane, is an organic compound with the formula C₂H₄O. This colorless, flammable gas has a faintly sweet odor and is the simplest epoxide. Due to its unique molecular structure, ethylene oxide readily participates in reactions. For instance, it can easily open its ring and engage in polymerization reactions. (Polymerization is a chemical reaction where small molecules bond together to form a larger molecule.)
Synthesis of Ethylene Oxide
Ethylene oxide can be produced by reacting 2-chloroethanol with sodium hydroxide. This reaction occurs at high temperatures, and instead of sodium hydroxide, compounds like potassium hydroxide, calcium hydroxide, barium hydroxide, magnesium hydroxide, or carbonates of alkali or alkaline earth metals can be used. Ethylene oxide can also be obtained by reacting calcium oxide with ethyl hypochlorite. Replacing calcium oxide with other alkaline earth metal oxides in this reaction decreases the yield. Industrially, ethylene oxide is produced by reacting ethylene with oxygen or by oxidizing ethylene. This reaction takes place in the presence of a silver-based alumina catalyst.
Ethylene oxide is one of the most widely used substances in the petrochemical industry and has numerous applications. Its primary use is in the production of ethylene glycols. It is also used in the production of plastics, ethanolamines, polyethylene glycols, polysorbates, glycol esters, ethoxylates, and acrylonitrile. Additionally, it serves as a disinfectant and sterilizing agent in hospitals and medical equipment.

Simulation of the Ethylene Oxide Unit in KHARK Pet. Co. with Aspen Plus
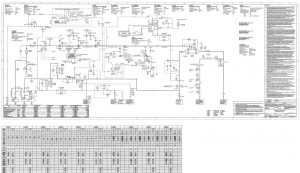
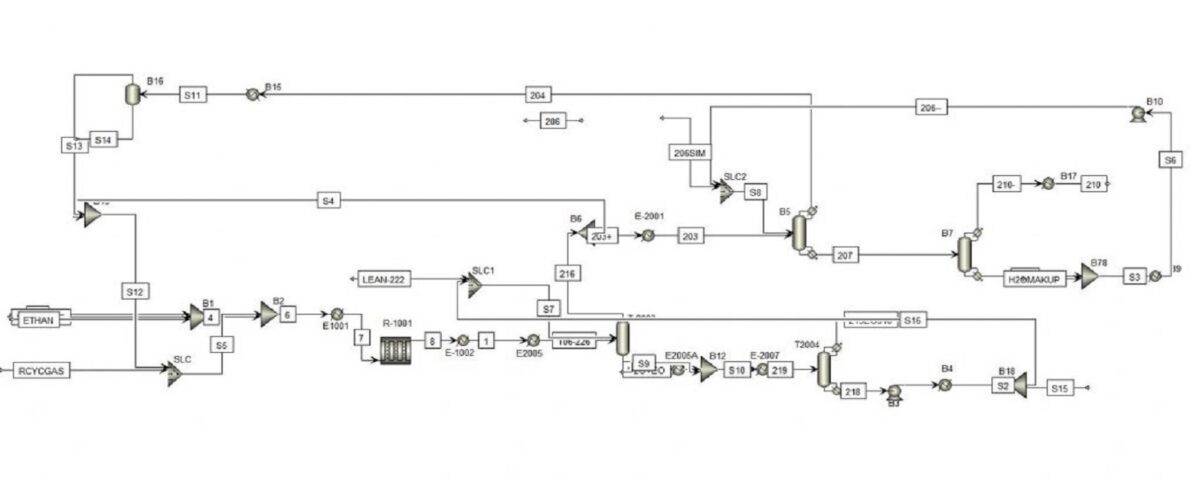
Ethylene oxide (EO) is produced through the catalytic oxidation of ethylene with oxygen over a silver catalyst. In this process, ethylene and oxygen are combined at a temperature of 200-250 degrees Celsius and a pressure of 10-15 bar, resulting in the production of ethylene oxide (EO), CO2, H2O, and small amounts of acetaldehyde and formaldehyde. The following figures depict the process flow diagram of the industrial ethylene oxide production unit at KHARG Pet. Co. Company. This project involves simulating the ethylene oxide unit of KHARG Pet. Co. Company using the Aspen Plus software.