Introduction
Oil refineries use a large amount of water for various purposes and produce significant amounts of wastewater. The characteristics of the produced wastewater are highly dependent on the process configuration. Pollutants such as cyanide, petroleum, phenols, benzene, sulfide, ammonia and heavy metals are present in refinery effluents.
In addition, chemicals are also found to control corrosion and biofouling. This wastewater usually consists of various types of organic and inorganic compounds that can be dangerous for the environment. Various methods were developed for the biological treatment of wastewater containing complex organic compounds.
Sour water removal process has emerged as an important process in pollution reduction practices. Because the regulation of waste water quality and energy saving has been emphasized. Therefore, the removal of chemical impurities such as hydrogen sulfide, carbon dioxide, and ammonia from the wastewater flow using the steam separation method has been the focus of researchers.
Description of The Process
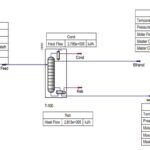
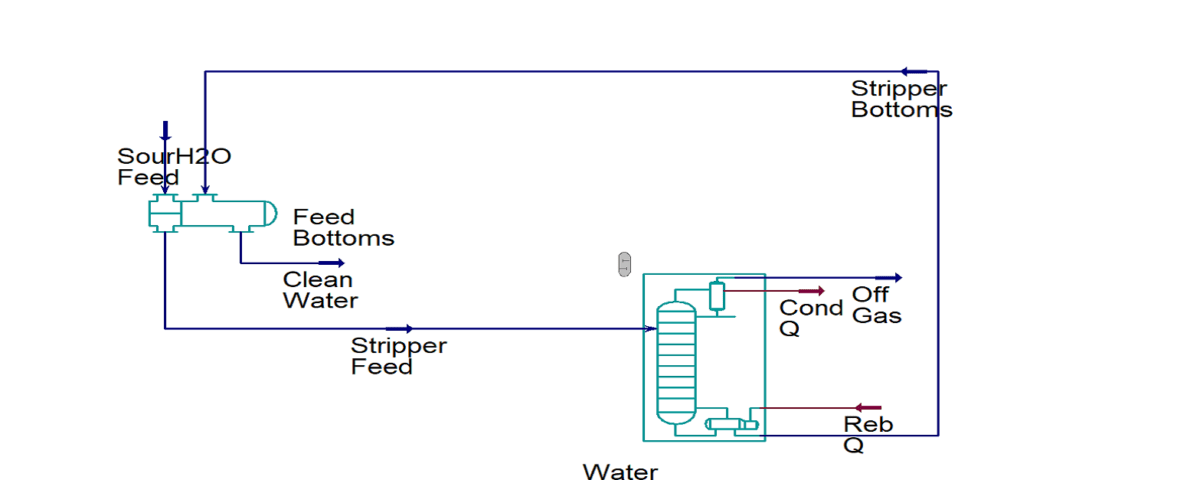
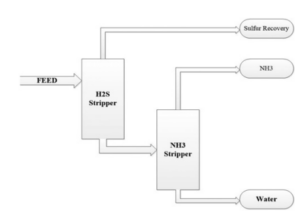
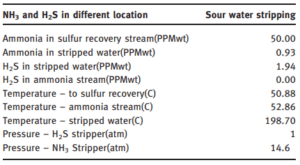
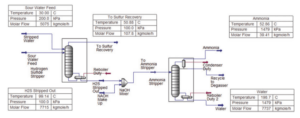
As shown in the figure below, the separation technique applies a two-stage stripping method to remove gaseous hydrogen sulfide (H2S) and ammonia (NH3) from sour wastewater. The first stage produces hydrogen sulfide, a superior feedstock to prescribed sulfur recovery units or sulfuric acid plants, and treated water that is suitable for reuse or discharge.
Then, in the second step, ammonia is separated. In general, CO2, H2S and HCN are treated in the hydrogen sulfide stripper. While NH3 is removed in the ammonia stripper. This is the main purpose for using two strippers because of the different characteristics of ammonia and hydrogen sulfide. Therefore, the main products in such a system are water, NH3 and sulfur. The operation of multistage H2S and NH3 stripping systems often involves reuse of the bottom discharge in later stages.
A process that gradually increases the concentration of H2S or NH3 in the bottom discharge. Based on a viable economic explanation, ammonia (NH3) can be recycled in several ways. This system was partially improved in response to the severe ecological constraints that factories were experiencing.
Process Simulation
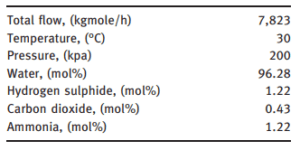
The following tables show the sour water feed conditions and the operating status of the sour water removal system, respectively. It was assumed that the system works under steady state conditions and heat and pressure drop is neglected. In this study, the thermodynamic/activity non-random two-fluid (NRTL) model was selected to be used as the feature package for the simulation.
Conclusion
The simulation results show that when the feed temperature is high in one stripper system and low in two striper systems. The stripping performance is increased. In both systems, the CO2 feed composition was low and no effect was observed for its relatively small amount.
The temperature of 70°C with the feed entering in the first stage for the H2S column and the temperature of 198°C with the feed entering in the fifth stage for the NH3 column and atmospheric pressure provided the optimal result in this study, which concluded that 100% ammonia in the two-striper system deleted However, this study presented an improved system that effectively restrained the column pressure and showed reasonable containment efficiency under constant pressure and at different temperatures.
Simulation of Separation of NH3 and H2S Gas From Water
In this project, the process of simulating the separation of ammonia and hydrogen sulfide gas from water has been simulated in Aspen Hysys version 14 software. The project is conceptually simulated. And it has an educational video.