Introduction
Given the environmental crises, the production of dimethyl ether (DME) as an environmentally friendly energy source has gained significant importance in the current industry. Although fossil fuels still dominate primary energy consumption worldwide, their use has drawbacks due to limited reserves and their contribution to air pollution. Therefore, biofuels have been extensively researched, produced, and utilized in recent years, as they offer a solution for reducing climate change and decreasing dependence on fossil resources.
Dimethyl Ether
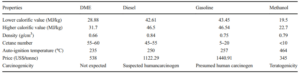
DME is a colorless, volatile, and non-corrosive gas that can be safely stored and transported, as it does not form explosive peroxides and does not release toxic particles and gases when burned. Dimethyl ether (DME) is an organic compound with the formula CH3OCH3, recognized as a clean fuel and a promising alternative to fossil fuels. The production of DME from sugarcane vinasse as a sustainable biomass source has attracted considerable attention.
The table below outlines the main characteristics of DME in comparison to other fuels.
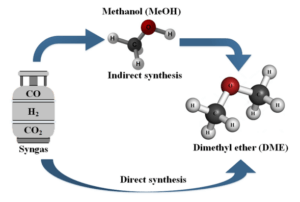
Synthesis of Dimethyl Ether
For The Synthesis of DME From Synthesis Gas (Syngas):
(1) Indirect synthesis, where methanol is produced as an intermediate and then converted to DME in a downstream reactor.
(2) Direct synthesis, where methanol and its dehydration to produce DME occur simultaneously in a single reactor.
The Production of DME From Synthesis Gas is Distinctly Divided Into Two Pathways:
(1) the indirect DME synthesis pathway, where DME is produced by dehydrating methanol generated in a separate reactor
(2) the direct synthesis of DME.
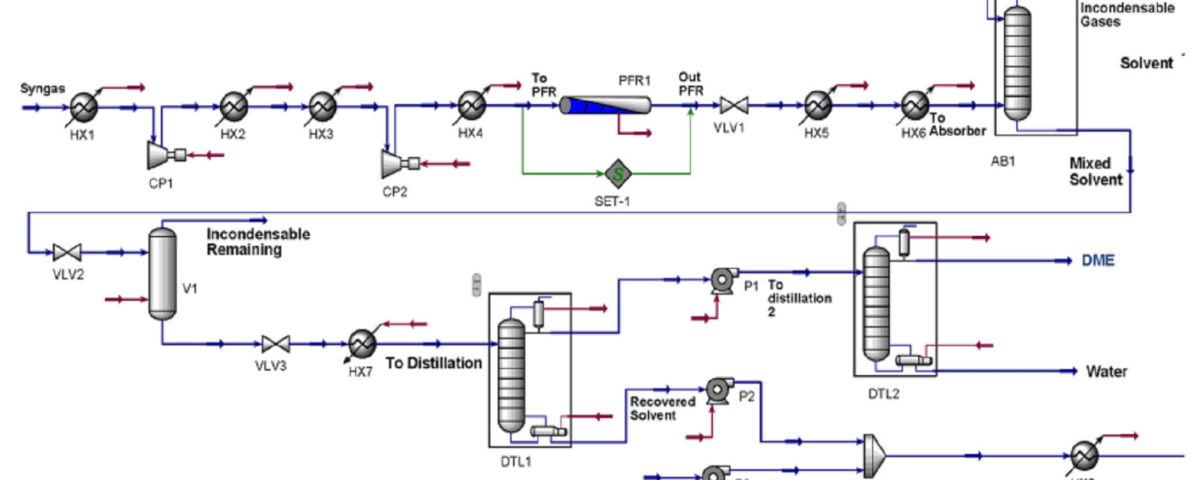
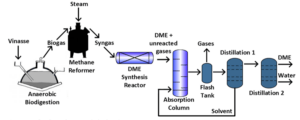
Process Modeling
In order to simulate the proposed model, a commercial process simulator was selected for process design, which provides a set of modeling, simulation and even process optimization tools with flexibility, reliability and robustness. with a mass flow rate of 66195 kg/h and a H2/CO molar ratio of approximately 2.8. Synthesis gas entered the process with a pressure of 199.6 kPa and a temperature of 410 K. The asymmetric thermodynamic model NRTL-RK was used based on the appointment of Matzen and Demirel.
Conclusion
This process is technically feasible and produces DME with the required quality specifications for widespread applications. Cost analysis indicates that the operational and capital costs for the gas synthesis to DME process are significant. Both processes, biogas to synthesis gas and synthesis gas to DME, have considerable costs and require the development of technology aimed at reducing these substantial expenses. Economic feasibility analysis showed that this pathway currently represents a viable alternative for reducing waste and generating profit in alcohol distillation plants.
Modeling and Simulation of Dimethyl Ether Production From Sugarcane Vinasse using Aspen HYSYS
In this project, the process of producing dimethyl ether from sugarcane vinasse has been simulated using Aspen HYSYS version 14.