Introduction
Carbon dioxide capture has become increasingly important in recent years. The significant release of carbon dioxide into the atmosphere, primarily from sources such as power plants, forest fires, and fossil fuels, has led to serious climate change issues like global warming and the increase of greenhouse gases. For instance, flue gases from power plants alone account for approximately 12% of the carbon dioxide in the atmosphere. This critical situation has highlighted the urgent need for effective methods to capture and separate carbon dioxide from the air.
Reactive Absorption Process with Monoethanolamine (MEA)
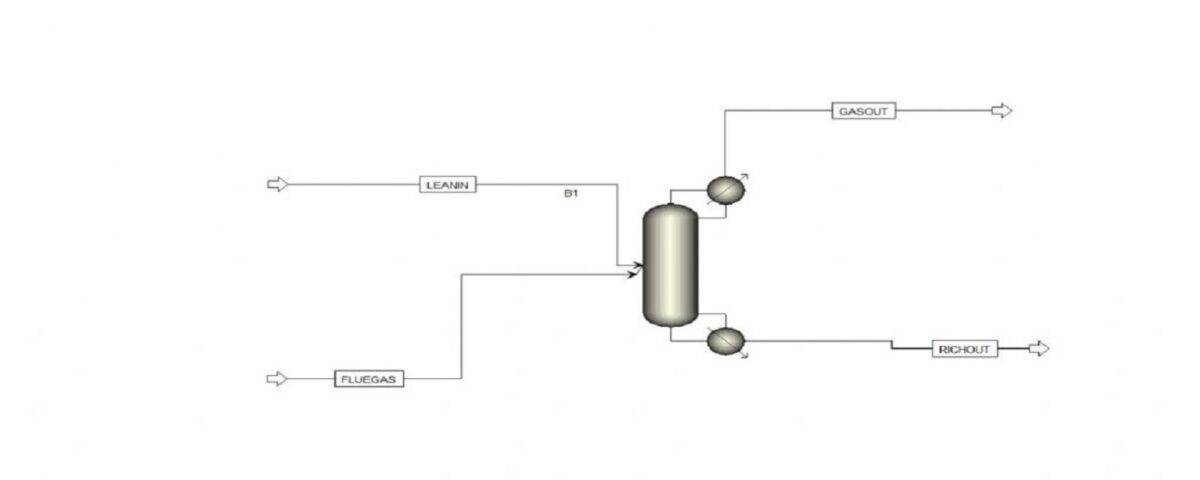
One of the most common industrial methods for removing carbon dioxide from flue gases is using chemical solvents like monoethanolamine (MEA). In this process, the gas containing carbon dioxide is passed through an aqueous solution of MEA. During this process, carbon dioxide reacts with MEA to form a new compound. Subsequently, the solution containing this compound is transferred to another tower, and by heating and reducing the pressure, carbon dioxide is separated from the solution, and MEA is recovered for reuse.

Absorbent Materials Used in Chemical Absorption Processes
A variety of absorbents are employed in absorption units, which can be categorized as follows:
- Amine solvents: Such as monoethanolamine (MEA) and diethanolamine (DEA). These are widely used due to their high reactivity with CO2.
- Dimethyl ether polyethylene glycol (dialkyl ether alcohol): A newer generation of solvents offering lower corrosivity and higher capacity compared to amines.
- Alkyl carbonates: Exhibit high absorption capacity and selectivity for CO2.
- Cold ammonia: Offers high absorption capacity but requires low temperatures for absorption and high energy for regeneration.
- Solid absorbents: Including activated carbons, ionic polymers, and zeolites. They are environmentally friendly and can be regenerated.
These absorbents are selected based on their properties such as low energy requirements, low volatility, high stability, selectivity for CO2, chemical resistance to impurities, and low production cost.
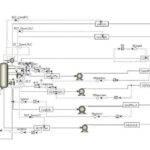
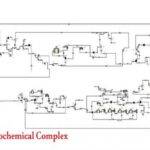
Currently, chemical absorption of CO2 using aqueous alkanolamine solutions is the most common commercial method for high-efficiency separation in the industry. In this project, the carbon dioxide absorption process using amine solvents was simulated based on two research papers. To view and download the papers, please click on the following link: [Insert link here]
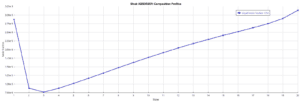
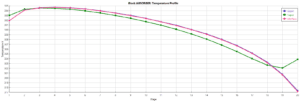
This simulation was conducted using Aspen Plus software. A portion of the results is presented below. The following figures show the profiles of CO2 percentage in the solvent used and the temperature profile.