Introduction
Distillation is one of the oldest and most widely used separation techniques in the chemical industries. Due to its high efficiency and simplicity, it continues to be employed in the production of high-purity materials. In the petrochemical industry, the separation of aromatic compounds such as benzene, toluene, and xylene (BTX) is of particular importance. These compounds serve as raw materials in the production of polymers, solvents, and other chemical products.
Benzene is the primary feedstock for many chemicals, including styrene and phenol. Toluene is used as a solvent and as a precursor in the production of benzene and para-xylene. Xylenes are utilized in the production of polyethylene terephthalate (PET) and as industrial solvents.
Correct selection of the distillation method and optimal design plays a crucial role in improving product quality and reducing operational costs. The use of modern technologies can enhance process efficiency and reduce energy consumption. Process optimization also contributes to mitigating environmental impacts.
Separation Process of BTX Aromatic Compounds
Benzene, toluene, and xylenes require precise design and optimization of distillation systems for effective separation due to their close boiling points and physical properties. This process is typically conducted using multiple distillation columns operating in succession to extract each component separately with high purity. Optimizing this process requires accurate simulation and the application of appropriate operational conditions.
Design and Simulation of the Distillation Process
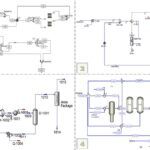
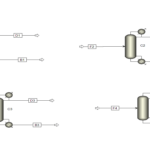
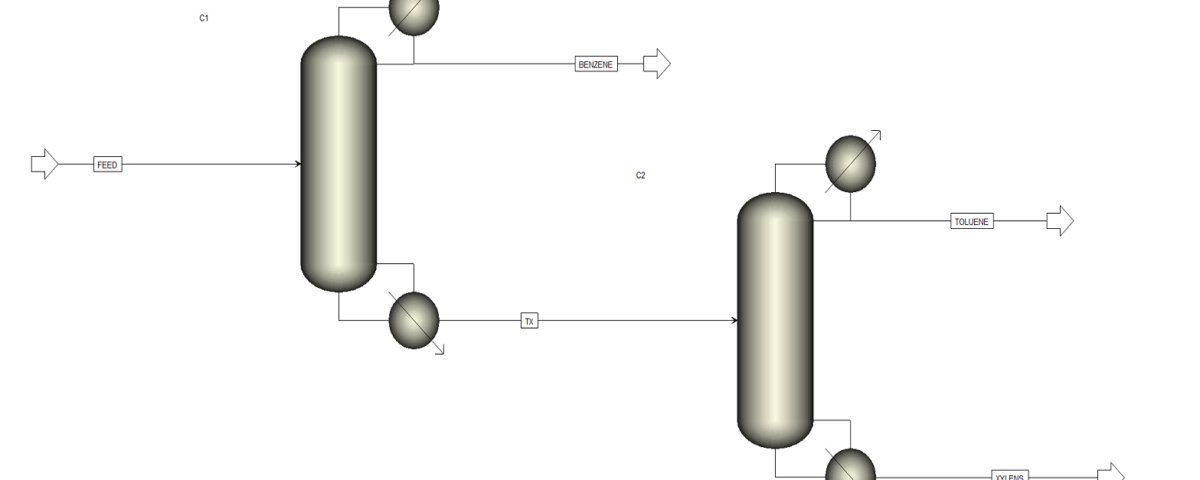
In this project, the simulation of the BTX separation process was carried out using Aspen Plus software. This software is one of the advanced tools for simulating chemical processes, enabling precise modeling and evaluation of various parameters. For this purpose, two distillation columns are employed in series:
– First Column: The primary goal of designing the first column is to separate benzene from the mixture. Benzene, due to its lower boiling point compared to toluene and xylenes, is separated in this column.
– Second Column: In this column, toluene is separated from xylenes. Toluene, having an intermediate boiling point, is removed from the mixture at this stage, while the xylenes remain in the downstream flow.
Operational Parameters and Optimization
Key operational parameters include the number of trays, reflux ratio, feed conditions (such as temperature, pressure, and composition), as well as the operating pressure of the columns. These parameters play a vital role in improving separation efficiency and reducing energy consumption. In this simulation, various configurations were tested for both columns, and optimal parameters were determined to achieve maximum possible purity with minimal energy consumption. The simulation results indicated that with appropriate parameter adjustments, energy loss could be minimized while maximizing efficiency.
Conclusion
The results of the BTX separation simulation demonstrate the high efficiency of this design and the optimization of operational parameters. This process, with high purity of final products and reduced energy consumption, can serve as a practical model for the design of industrial distillation units. This research shows that precise simulation and proper optimization can lead to cost reduction, improved efficiency, and enhanced product quality in the petrochemical industry.
Simulation of the BTX Separation Process in Aspen Plus
In this project, the simulation of the separation process of aromatic compounds benzene, toluene, and xylenes (BTX) was carried out using Aspen Plus software based on methods and data from a research article, and focused on analyzing and optimizing operational parameters.