Introduction
Biodiesel is a type of fuel produced from the processing and modification of vegetable oils. Introduced in the 1970s as a substitute for petroleum diesel, it initially did not gain popularity. However, today, with the diminishing oil resources and the increasing demand for various fuels, the use and production of biodiesel have gained significant attention and importance.
The molecular size of biodiesel and petroleum diesel is similar, but the two fuels differ in their chemical structure. Biodiesel molecules are almost entirely composed of methyl esters of fatty acids, which contain unsaturated aliphatic compounds. In contrast, 95% of petroleum diesel (low sulfur) is made up of saturated hydrocarbons, with the remaining 5% consisting of aromatic compounds.
If ethanol is used instead of methanol in biodiesel production, the resulting molecules will be ethyl esters of fatty acids. The chemical differences in the structure of these two types of fuels also result in variations in their physical properties, which will be highlighted later in terms of advantages and disadvantages.

Biodiesel Production Process
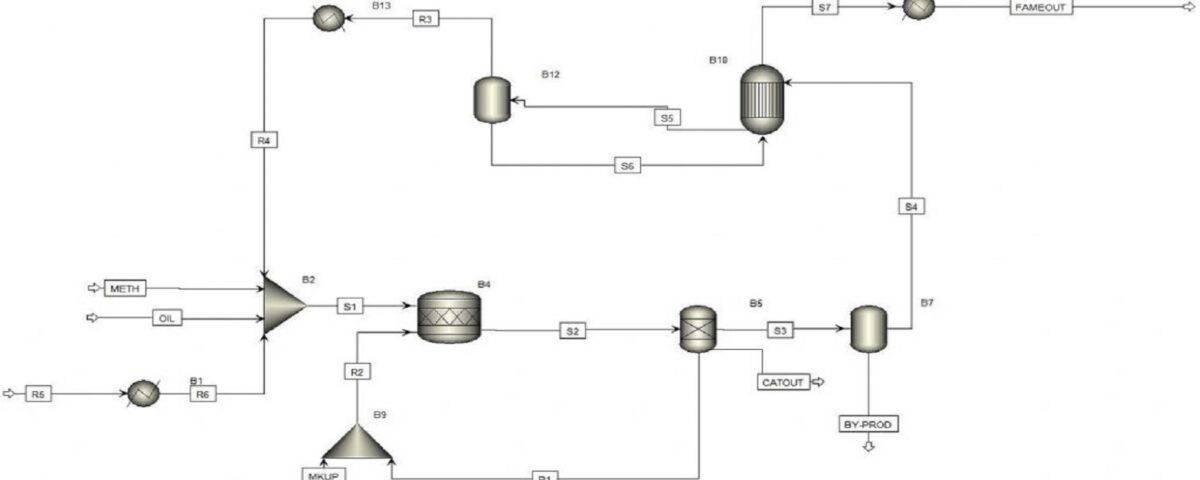
Biodiesel can be produced from vegetable oils, yellow grease, cooking oils, or animal fats. This is achieved through a process called transesterification, which yields biodiesel and glycerin as products. Approximately 100 parts (by weight) of oil or fat react with 10 parts of a short-chain alcohol (usually methanol) in the presence of a catalyst (typically sodium hydroxide or potassium hydroxide) to ultimately produce 100 parts of biodiesel and 10 parts of glycerin.
Simulation of the Biodiesel Production Process
In this project, the simulation of the biodiesel production process has been carried out using Aspen Plus software.