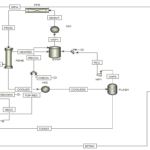
Introduction
Nitric acid (HNO₃) is one of the most widely used and important mineral acids in the chemical industry, holding a significant position in industrial processes due to its unique properties. This colorless or pale yellow compound has a sharp odor and high corrosiveness. As a strong oxidizing agent, nitric acid has the ability to react with metals, organic and inorganic materials, which is why it is utilized in the production of many chemical products.
The history of nitric acid usage dates back to the Middle Ages when alchemists produced it by heating metal nitrates and alum. With the advancement of chemistry, modern and industrial methods for producing this acid were developed. The Ostwald process, introduced by Wilhelm Ostwald in 1902, is now recognized as the standard method for industrial nitric acid production. This method uses ammonia (NH₃) as a raw material and efficiently produces nitric acid through catalytic reactions and gas absorption.
Simulation and modeling of nitric acid production units are powerful tools for gaining a deep understanding of processes and improving their performance. By utilizing mathematical models and computer simulations, process parameters can be analyzed, improvement points identified, and various operational scenarios evaluated. This enables managers and engineers to make more informed decisions to optimize the process.
Description of The Nitric Acid Production Process
The production of nitric acid (HNO₃) is one of the most important chemical processes in the industry, holding special significance due to its wide applications in the production of fertilizers, explosives, and other chemical industries. The nitric acid production process typically involves several stages, each requiring high precision and control of specific conditions.
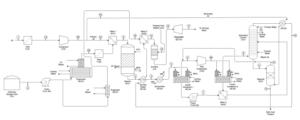
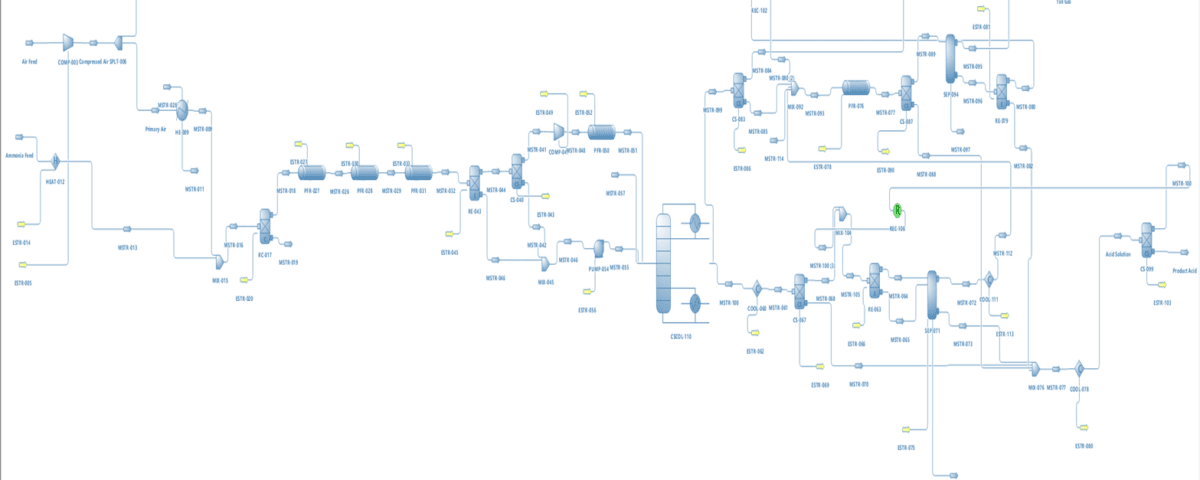
Nitric acid production process consists of four main stages: ammonia oxidation, nitrogen monoxide oxidation, absorption, and reduction of nitrogen oxides.
Ammonia Oxidation
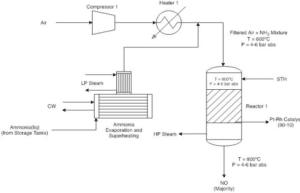
Ammonia oxidation is the first and most crucial step in the production of nitric acid. In this stage, ammonia (NH₃) is oxidized to nitrogen monoxide (NO) in the presence of oxygen (O₂) and using a catalyst, typically platinum or rhodium. This reaction occurs at high temperatures (around 800 to 900 degrees Celsius).
In this process, the production of water as a byproduct can significantly impact the overall efficiency of the system, necessitating precise water management. Generally, this stage is one of the most critical parts of nitric acid production, as temperature and pressure conditions can directly influence the conversion rate of ammonia to nitrogen monoxide. Therefore, careful control of operational parameters is essential for optimizing yield and preventing the formation of unwanted byproducts. Additionally, safety must be a top priority at this stage, as ammonia is a toxic and hazardous gas.
Nitrogen Monoxide Oxidation
In the next stage, nitrogen monoxide (NO) produced in the ammonia oxidation step is oxidized to nitrogen dioxide (NO₂). This reaction takes place using oxygen at various temperatures and under appropriate pressures. Nitrogen dioxide is recognized as an essential compound in the production of nitric acid and can serve as a raw material for subsequent stages in this process.
The significance of this stage lies in the fact that the production of NO₂ can act as a loop for returning to previous stages, assisting in the optimization of the overall process. Additionally, NO₂ can react with water to form nitric acid. At this stage, careful control of temperature and pressure is particularly crucial, as it has a direct impact on the rate of production and the quality of the final products.
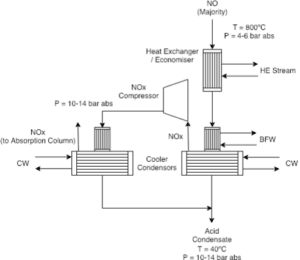
Absorption
After the production of nitrogen dioxide, the absorption stage begins. In this phase, the produced NO₂ gas is absorbed into an aqueous solution. This process typically occurs in absorption towers, where NO₂ reacts with water to generate nitric acid (HNO₃). During this stage, NO is released as a byproduct, which can be returned as a secondary compound to previous stages.
This stage of the nitric acid production process is highly significant as it can have a direct impact on the purity of the produced nitric acid. Additionally, the quality of the water used in this process must also be taken into consideration, as impurities can affect the final quality of the nitric acid.
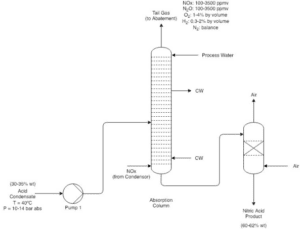
Reduction of Nitrogen Oxides
In the final stage, nitrogen oxides (NO and NO₂) may be reduced in order to mitigate environmental impacts and minimize greenhouse gas emissions. This process is typically carried out using catalysts and under specific conditions. During this stage, nitrogen oxides are converted into nitrogen (N₂), which is recognized as a harmless gas for the environment.
This stage acts as a supplementary step in the nitric acid production process and can help reduce the negative environmental effects associated with the production and use of nitric acid. Additionally, this stage can lead to the optimization of industrial processes and enhance energy efficiency.
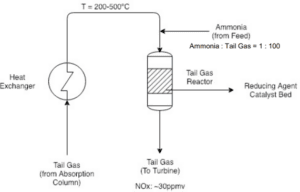
Important Points in This Process
In the nitric acid production process, precise control of temperature and pressure at each stage is crucial. These parameters must be maintained within appropriate ranges to enhance process yield and produce a high-quality product. Continuous monitoring of the levels of impurities present in both raw materials and the final product is also essential. The presence of impurities can lead to a reduction in quality and process efficiency. Therefore, impurities should be minimized using filters and other purification methods.
Other important considerations include the appropriate selection of materials and equipment used in the process. The materials should be environmentally compatible and safe, and the equipment must be carefully designed and constructed to prevent potential issues.
Optimizing the nitric acid production process through improving the efficiency of various stages, reducing energy and raw material consumption, and increasing the overall yield can lead to enhanced profitability and competitiveness of the production unit. In addition to technical aspects, environmental and safety issues must also be taken into account. Proper discharge of wastewater, waste management, and adherence to safety standards are of utmost importance.
Overall, the nitric acid production process is a complex, multi-stage operation that requires precision and careful control of various parameters. Improvements and optimizations in this process can lead to increased efficiency, reduced costs, and environmental protection.
Advantages and Disadvantages of The Nitric Acid Production Process
In general, the nitric acid production process has its advantages and disadvantages:
Advantages:
1. High Efficiency: The nitric acid production process, particularly in medium-pressure systems, has a high yield in converting ammonia to nitric acid. This process effectively increases the amount of acid produced.
2. Cost Reduction: The use of catalysts such as platinum-rhodium reduces the need for frequent changes in catalysts, thereby lowering operational costs. This characteristic minimizes downtime and saves on maintenance expenses.
3. High Stability and Reliability: The medium-pressure process exhibits high stability and requires less maintenance, which is especially important in large industries.
4. Flexibility in Production: This process allows for the production of nitric acid at various concentrations for different industrial applications.
Disadvantages:
1. Environmental Pollution: One of the major disadvantages of the nitric acid production process is the emission of nitrogen oxides (NOx), which can lead to air pollution and environmental hazards. This issue must be addressed in many production units through exhaust gas treatment systems.
2. High Energy Costs: The nitric acid production process requires high energy consumption, particularly during the evaporation and heating stages of the gases. This can increase production costs.
3. Damage to Equipment: The gaseous vapors and chemicals used in the production of nitric acid can cause erosion and damage to industrial equipment, necessitating frequent repairs and replacements.
4. Water Management Issues: The nitric acid production process requires precise water management. The presence of excess water in liquid ammonia can lead to problems in the evaporation process and reduce the quality of the final product.
Simulation and Optimization of Nitric Acid Production
Simulating the nitric acid production process in DWSIM software is an effective method for modeling and analyzing the performance of this chemical process. DWSIM is an open-source chemical process simulation software that allows users to design and optimize industrial processes. The following outlines the steps for simulating the nitric acid production process in DWSIM:
1. Defining The System:
– Inputs: To begin the simulation, the raw materials required for nitric acid production must first be identified. These materials typically include ammonia (NH3) and oxygen (O2).
– Final Product: The ultimate goal of the simulation is to produce nitric acid (HNO3).
2. Simulation Settings:
– Thermodynamic Model: Choose an appropriate thermodynamic model (such as Peng-Robinson or Soave-Redlich-Kwong) to calculate the physical and chemical properties of the materials.
– Defining Units: Define the main units such as reactors, heat exchangers, distillation columns, and other necessary equipment.
3. Modeling The Reaction:
– Chemical Reactions: The simulation includes defining the chemical reactions involved in nitric acid production. For example, the oxidation of ammonia to nitrogen dioxide (NO2) and then the conversion of that to nitric acid.
– Reaction Parameters: Set the reaction parameters such as temperature, pressure, and catalysts.
4. Designing and Implementing Units:
– Reactor: Design a reactor where the chemical reactions take place. This may include continuous or batch reactors.
– Separation Systems: Design separation units such as distillation columns for separating by-products and ensuring the purity of nitric acid.
5. Analysis and Optimization:
– Performance Analysis: After the simulation, analyze the results and evaluate the performance of the system. This includes examining temperature, pressure, production rate, and the purity of the final product.
– Optimization: Using DWSIM’s optimization tools, you can enhance the process to improve yield and reduce costs.
6. Reporting and Documentation:
– Results Reporting: DWSIM allows you to document the simulation results in the form of graphical and numerical reports.
– Economic Analysis: Review the production costs, pricing, and conduct an economic analysis of the process.
7. Testing and Validation:
– Validation: Compare the simulation results with experimental data or existing information to confirm the accuracy of the model.

UHDE Company
UHDE is a pioneer in the chemical process and engineering industry, particularly focused on the design and optimization of processes. This company, which is part of the Thyssenkrupp group, has a long history of providing innovative technologies and engineering solutions for various industries, including nitric acid production.
The UHDE license for nitric acid production is a technology permit that allows companies to utilize the advanced knowledge and technologies of UHDE in the nitric acid production process. This license includes a set of technologies, designs, and operational methods that assist in optimizing production stages and enhancing efficiency.
Key Components of The UHDE License
Advanced Technologies
The UHDE license encompasses innovative and up-to-date processes in the areas of catalysts, reactions, and process designs that contribute to increased productivity and cost reduction.
Production Unit Design
This license includes precise design and engineering of production units, enabling companies to establish optimized and efficient production facilities.
Process Management and Control
Advanced control systems for monitoring the production process and optimizing operational conditions are part of this license. These systems help reduce errors and enhance the quality of the final product.
Safety and Environmental Standards
The UHDE license places significant emphasis on compliance with safety standards and the reduction of emissions. This includes techniques for reducing greenhouse gas emissions and other pollutants.
Training and Support
UHDE typically provides necessary training and technical support to companies receiving the license to ensure that the technology is implemented correctly.
Innovation and Upgrading
This license allows companies to leverage new innovations and technology updates in production, ensuring they remain at the forefront of the industry.
Overall, the UHDE license for nitric acid production represents a comprehensive and complete set of technologies and methods that assist companies in optimizing their production while adhering to safety and environmental requirements. This license is considered a key tool in the chemical industry, enabling manufacturers to operate effectively and efficiently.
Anil Pars Process Industry Company
Anil Pars Process Industry Company proudly operates as one of the leaders in the field of process design, simulation, and optimization. Leveraging the expertise and experience of its human resources, the company has the capability to undertake various projects in the domains of water and wastewater treatment, industrial process optimization, and the design of diverse systems.
One of the company’s standout features is its proficiency in specialized software related to process simulation. In particular, Anil Pars Process Industry Company is capable of simulating and optimizing nitric acid production processes using the DWSIM software based on the UHDE license.
We take pride in our continuous efforts to provide innovative and effective solutions aimed at enhancing quality and improving industrial performance.
Examples of Projects Completed by APIPCO
Feasibility Study with Aspen Plus and HYSYS Simulation of Nitric Acid Production Process
Simulation and Optimization of The Nitric Acid Production Process for Thyssenkrupp UHDEH Using DWSIM Software
In this project, the simulation and optimization of the nitric acid production process for Thyssenkrupp UHDEH has been conducted using DWSIM software. The project includes a comprehensive report.