Introduction
The increasing global demand for natural gas requires not only the improvement of existing processes for energy production and consumption but also effective pollution control. One of the most significant challenges faced by technologies related to natural gas processing is the presence of high amounts of acid gases, which constitute approximately 40% of the gas output from wells. About 13.46% of natural gas reservoirs worldwide contain H2S levels exceeding 10%, while around 26.9% of these reservoirs have CO2 concentrations greater than 10%.
Various methods have been proposed to reduce the levels of acid gases in natural gas, including chemical absorption, physical absorption, hybrid processes, adsorption using solid columns, and membrane utilization. Among these methods, the absorption of acid gases by a liquid solvent (chemical absorption) is the most commonly used approach in the gas sweetening industry.

Natural Gas Sweetening
In the 1930s, alkanolamines were first used for natural gas sweetening, and from then until the 1970s, monoethanolamine (MEA) was the most widely used. However, due to the disadvantages of MEA, such as corrosion and solvent loss, diethanolamine (DEA) replaced it after the 1970s. Since the mid-1970s, particularly in the last two decades, methyl diethanolamine (MDEA) has gained extensive use in the gas industry due to its advantages, such as the ability to selectively separate hydrogen sulfide in the presence of carbon dioxide, high stability, and low energy consumption for solvent recovery.
Alkanolamines generally consist of at least one hydroxyl group (-COH) and at least one amino group (-NH2). The commonly used amines for gas sweetening include:
– Primary amines: Monoethanolamine (MEA) and diglycolamine (DGA)
– Secondary amines: Diethanolamine (DEA) and diisopropanolamine (DIPA)
– Tertiary amines: Triethanolamine (TEA) and methyl diethanolamine (MDEA)
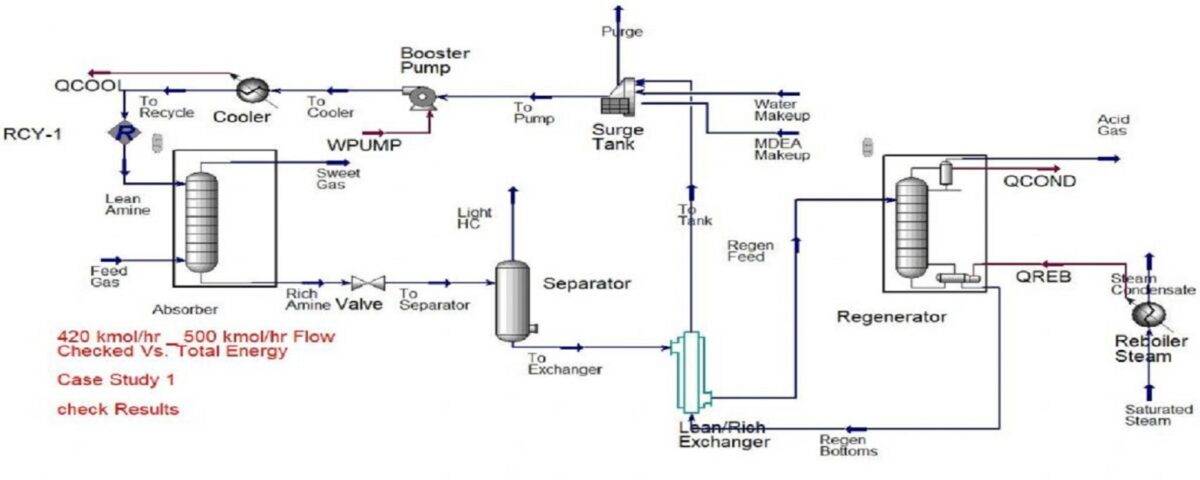
Simulation and Optimization of Natural Gas Sweetening Unit with MDEA
In this project, the natural gas sweetening unit using MDEA has been simulated and optimized in Aspen HYSYS software.
Methyl diethanolamine is classified as a tertiary amine and therefore lacks active amino groups (-NH) in its molecular structure. As a result, it has a stable chemical structure and selective capability for absorption. The primary cause of degradation in primary and secondary amines is the presence of these active amino groups. The degraded amine is typically highly corrosive.
This stability of the tertiary amine allows for concentrations as high as 50% to be used without concerns related to corrosion. This means that a given amount of acid gas can be absorbed with a lower flow rate of the tertiary amine solution compared to the required flow rates for primary or secondary amine solutions, resulting in a significant reduction in the thermal energy consumption by the reboilers in the regeneration tower.