Introduction
In recent decades, rapid population growth and industrial development, coupled with rising living standards, have significantly increased the demand for energy resources. According to BP’s 2019 Energy Outlook, the global energy demand is projected to increase by approximately 1.2% annually. One of the main issues in this regard is the rising carbon dioxide emissions and global warming.
Natural gas is gaining attention as a crucial energy source for the future due to its high efficiency and lower greenhouse gas emissions compared to oil. The EIA reports suggest that costs associated with natural gas will remain low until 2050, making it an economical and effective option for hydrogen production. On the other hand, the abundant reserves of coal and its efficient performance in power generation and hydrogen production indicate that coal will continue to be an important energy source.
Given the increasing energy needs and the necessity to reduce greenhouse gas emissions, technologies such as coal gasification and steam methane reforming (SMR) have emerged as viable options for hydrogen production. These processes provide cost-effective solutions for meeting future energy demands by enabling the production of hydrogen and other products.
To actualize new projects, conducting feasibility studies is essential. These studies contribute to resource allocation optimization, risk reduction, and effective financial planning. This project focuses on feasibility studies for methanol production from carbon dioxide, considering the location in the Khuzestan special economic zone to reduce tax costs.
Complete Methanol Synthesis Process
General and Physical Properties
Methanol (CH3OH), also known as methyl alcohol or wood alcohol, is the simplest alcohol. This colorless liquid has an alcoholic odor and emits a pungent scent in its raw state. It is highly polar and flammable, with a flammability range of 6% to 36% by volume in air. Methanol reacts violently with oxidizers and is toxic, causing irritation to the eyes and skin. It readily mixes with water, alcohol, ethers, benzene, and ketones.
History of Production
Initially, methanol was primarily produced through the destructive distillation of wood, a method used from the mid-19th century to the early 20th century. The development of methanol synthesis from hydrogen and carbon oxides in the 1920s rendered this method obsolete. In the 1920s, BASF company conducted the first commercial methanol synthesis using a zinc oxide-chromium oxide catalyst, marking the beginning of high-pressure production technology.
Production Methods
Industrial-scale methanol production is mainly carried out from a gas mixture of hydrogen, carbon dioxide, and carbon monoxide in the presence of a heterogeneous metal catalyst. The synthesis gas pressure depends on the catalyst activation used. Production processes are categorized into three pressure ranges: low (5-10 MPa), medium (10-25 MPa), and high (25-35 MPa).
Common Technologies
Today, there are three major processes for methanol production worldwide: ICI, Lurgi, and Mitsubishi. The main differences between these processes lie in reactor design and heat removal methods. In the ICI process, the reactor is composed of several adiabatic fixed catalyst beds, using cold feed gas to cool the reactors between the beds. In contrast, the reactors designed by Lurgi and Mitsubishi operate nearly isothermally, reducing catalyst deactivation.
Applications of Methanol
Methanol is a highly important product in the chemical industry and is used in the production of many materials. Some of its industrial applications include:
– Production of various industrial adhesives
– Use in solvents and antifreezes
– Production of plastics, plywood, paints, and explosives
– Production of methyl methacrylate for laminates and varnishes
– Use in manufacturing kitchen utensils and equipment
– Production of formaldehydes for household uses and resin production
– Production of MTBE to improve the octane rating of gasoline
– Use of dimethyl ether from methanol as a substitute for CFCs in aerosol sprays
– Production of acetic acid either as a final product or for various cellulose acetate synthesis
Given the anticipated shortage of energy resources in the future, the use of methanol as a clean fuel or in hydrogen production for fuel cells is gaining considerable attention.
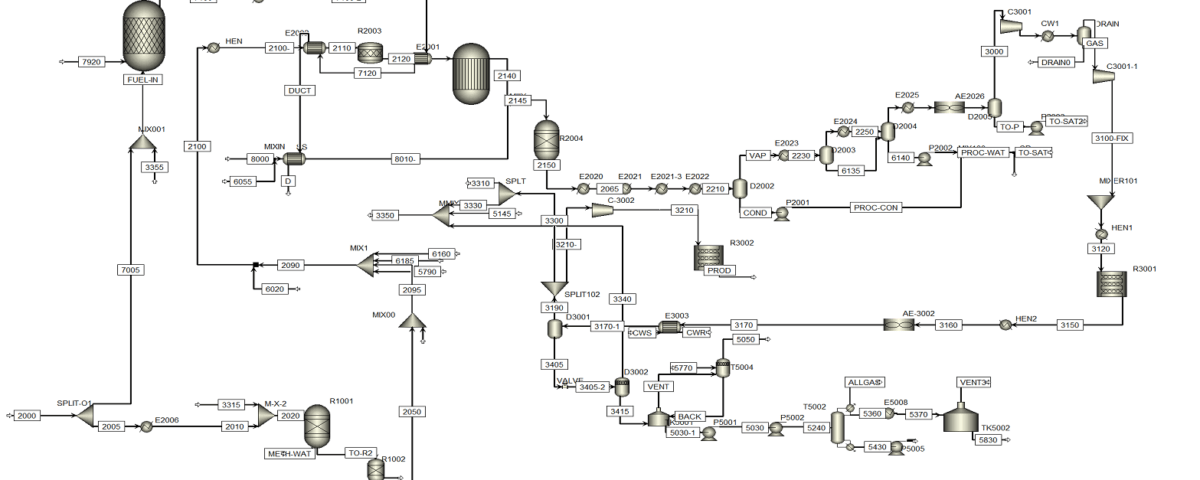
Methanol Production Process Description
Production Stages
Methanol production primarily occurs through its synthesis using carbon monoxide and hydrogen gases. This process can be executed at varying pressures, including both high and low pressures. Currently, the low-pressure process, due to its lower costs, is more common. There are two patents for low-pressure methanol production named Lurgi and ICI, offering diverse methods.
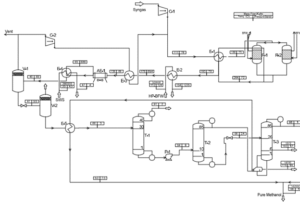
The overall methanol production process generally involves four main stages:
1. Desulfurization: In this stage, sulfur compounds present in natural gas are converted to hydrogen sulfide and absorbed using adsorbents like activated carbon, reducing sulfur compound levels to around 0.2 ppm.
2. Cracking: Natural gas along with steam enters a reactor filled with nickel catalyst. At approximately 870 degrees Celsius and 20 atmospheres, synthesis gases including carbon monoxide, carbon dioxide, hydrogen, and methane are produced.
3. Synthesis: The output gases from the cracking stage are cooled and compressed before being transferred to the synthesis reactor. In this stage, the carbon monoxide and carbon dioxide gases react with hydrogen to produce methanol. The catalyst used consists of a combination of copper, zinc, chromium, and aluminum salts, and the reaction occurs at around 240 degrees Celsius and 100 atmospheres.
4. Purification: Methanol is separated from the reactor’s output mixture, and after passing through a distillation system, other compounds like dimethyl ether, acetone, methyl acetate, and ethanol are separated, yielding methanol with a purity of 99.9%.
Synthesis Cycle
In the methanol production process, synthesis occurs at approximately 70 bar pressure and 220 degrees Celsius using a copper/zinc catalyst. The reactions in this process are equilibrium-based, meaning only a portion of the compounds in the synthesis gas reacts, while the remainder exits the reactor unchanged. To maximize methanol production, it is necessary to separate the existing methanol from the reactor’s output gas, with the remainder of the gas being recycled back into the reactor.
The synthesis cycle unit comprises various components, including:
– Synthesis reactors
– Circulators
– Air coolers and water coolers
– Raw methanol separators
– Water preheaters for the saturation system and gas exchangers
The output gas from the synthesis compressor is divided into two parts; one part, after mixing with the output gas from the drum and heating in the exchanger, is directed to the reactor. The synthesis reactor’s output is cooled down to 45 degrees Celsius in air coolers and water coolers, where the methanol in the gas is condensed and separated in the raw methanol drums.
Economic Calculations of the Process Unit
In the economic calculations section, we first assess the energy consumption in the methanol production process using Aspen Plus software and the Aspen Energy Analyzer (AEA) module. This assessment allows us to accurately identify the necessary ancillary energy sources and perform the required optimizations. The graph below illustrates the ancillary energy consumptions in the process unit.
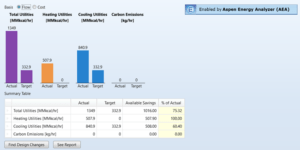
After completing the assessments, the software calculates the annual costs associated with the energy consumption. This information is obtained in the AEA section of the software and is presented in the following graph, showcasing the annual energy costs. These calculations play a crucial role in economic analysis and process optimization, as accurately determining energy costs helps improve efficiency and reduce the operational costs of the process unit.
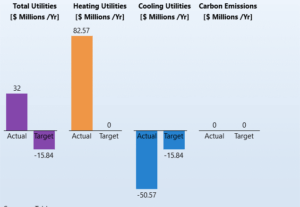
Construction and Installation Cost Calculations of Process Equipment
In the economic evaluation of the methanol production process, the costs associated with the construction and installation of main equipment play a crucial role in the overall analysis. Key equipment considered in these economic calculations include:
– Reforming Reactor Furnace: This furnace plays a vital role in the natural gas reforming process. The choice and installation of it are crucial based on operational needs and process efficiency.
– Reforming, Desulfurization, and Methanol Synthesis Reactors: These reactors perform different stages of the process and must be accurately chosen and designed. Proper installation of these reactors is essential for ensuring optimal process performance.
– Heat Exchangers: Heat exchangers are used to transfer heat between different streams in the process. The costs associated with purchasing and installing these exchangers vary based on their type and application.
– Compressors and Pumps: These pieces of equipment are used for moving and compressing gases and liquids in the process. Choosing appropriate compressors and pumps with the necessary capacity and operational pressure is a significant part of the economic calculations.
– Distillation Towers and Two-Phase Separators: Distillation towers are used to separate products and reaction mixtures at various stages of the process. Additionally, two-phase separator tanks (vessels and storage tanks) are employed for storing and separating products.
The costs associated with this equipment include purchasing, installing, and commissioning costs, calculated accurately based on operational needs and process conditions to be included in the economic analysis.
Pricing of Reactors Used in the Overall Process
Estimating the prices of reactors used in the methanol production process, especially catalytic reactors, is achieved through a relatively straightforward method. First, assuming a residence time of 0.02 hours for the feedstock, which is deemed a suitable duration, the volume of the reactor is calculated. After determining the reactor volume, its price is estimated based on this volume.
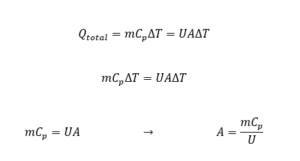
It is worth mentioning that the estimated price at this stage does not include costs related to the catalyst. The catalyst costs are calculated separately based on available resources from foreign sites. Considering the unit’s capacity and as an industrial unit, this price is also estimated.
This method serves as an initial approach for assessing capital investment costs in catalytic reactors and can support financial and economic decision-making in the early stages of process design and development.
The reactors present in the process are listed in the table below:
Prices of Heat Exchangers, Coolers, and Heaters in the Overall Unit
Pricing thermal equipment like heat exchangers, cooling systems, and heaters is typically determined based on the heat transfer surface area between fluids. To determine this surface area, it is necessary to write heat balance equations around the equipment. In this project, the energy balance for the equipment is written as follows:
Thus, the contact area can be calculated using mass flow rates, the specific heat capacity of the fluid, and the overall heat transfer coefficient. Once the contact area is determined, the prices for thermal equipment can be estimated using empirical relationships and economic databases for industrial equipment.
Note: Accurate pricing requires more information such as the type of exchangers, construction materials, operating conditions, and related installation and maintenance costs.
Distillation Towers and Two-Phase Separator Tanks (Vessels and Storage Tanks)
Distillation towers and two-phase separator tanks are crucial equipment in industrial processes. Estimating their prices is usually based on the weight of the shell. To perform this estimation, information such as tower diameter and height is required. These specifications directly affect the weight and, consequently, the cost of manufacturing and installation.
In this project, the internal design of the towers is conducted using the INTERNAL software, allowing precise extraction of dimensions by defining details such as tower type, number of trays, packing type, and other operational parameters. After determining these dimensions, the shell weight can be calculated, and associated manufacturing and installation costs can be estimated.
Estimating the costs of towers and storage tanks not only includes prices for raw materials (such as stainless steel or carbon steel) but also considers other costs such as manufacturing, transportation, installation, and insulation. Therefore, using design and process analysis software plays a significant role in optimizing and reducing costs.
Total Investment Calculations
Total investment calculations involve several parameters critical to industrial processes. These parameters include instruments, piping, landscaping, and other ancillary equipment. To accurately estimate the investment, besides the main costs related to processing equipment, these auxiliary costs must also be considered.
To calculate these costs, tables such as the Total Capital Investment (TCI) table are used. These tables are usually estimated based on the type and scale of the project, providing costs for various sections. For example, the cost of instrumentation may be estimated as a percentage of the primary equipment costs, whereas piping and landscaping costs are estimated based on footage and the types of materials used.
In this project, using the TCI table, the overall costs related to investments have been calculated. This method allows for a comprehensive view of the investment needed to set up and operate the process unit. These estimates are crucial for economic decision-making and budgeting across different project sections.
Conclusion
The adoption of alternative fuels in various energy-intensive industries not only enhances the efficiency of energy resources but also contributes to optimal allocation of these resources, energy security, and sustainable environmental development. This study explores a process design for the simultaneous production of methanol, hydrogen, and power. The results indicate a methanol production rate of approximately 16 tons per hour, hydrogen production of around 4 tons per hour, and a net power output from the process of about 45 megawatts. This production level coupled with an estimated annual net profit of approximately $62 million showcases the economic viability of the project.
With a unit cost in the base case of about $10 million per year, the estimated costs are $300 per ton of methanol, $2 per kilogram of hydrogen, and about 3 cents per megajoule for power. These prices, compared to current product prices ($550 per ton of methanol, $10 per kilogram of hydrogen, and 10 cents per megajoule of power), indicate competitive capability in the market. Moreover, conducting feasibility studies for any business is essential, as it can eliminate unfeasible projects and save costs and time.
Methanol is one of the key products in the petrochemical industry in Iran. This project centered on the feasibility study of methanol production from CO2 through hydrogenation—considered the most suitable method. Additionally, it evaluated the simulation of the methane reforming unit, synthesis, and its economic calculations and investment costs. The results indicate that the proposed process possesses suitable economic and technical potential, which can contribute to sustainable development in the petrochemical industry.
Technical and Economic Study of the Natural Gas Reforming Process for Methanol Production with Complete Process Simulation Using Aspen Plus Software
This project, which is part of the practical activities of the plant design and economics class, evaluates the technical and economic study of the natural gas reforming process and methanol production along with its feasibility evaluation and complete process simulation using Aspen Plus version 12.
Simulation of Natural Gas Reforming Process and Methanol Production in Aspen Plus
In this project, the simulation of the natural gas reforming process and methanol production has been conducted using Aspen Plus version 12.