Introduction
The purpose of this project is to build a chemical plant that produces ethyl acetate which will be used as a solvent in synthetic fragrance products. There are various process intensification techniques used for ethyl acetate production including the Fischer Esterification, ethanol dehydrogenation, and direct addition of acetic acid to ethylene.
The effect of different process parameters has been discussed and the results showed that the ethanol dehydrogenation process is the most suitable process for the production of ethyl acetate. Based on the global production capacity of 3.2 million tons per year and the growing demand that may reach 4.9 million tons per year, the market gap is about 2.5 million tons per year.
Ethyl Acetate
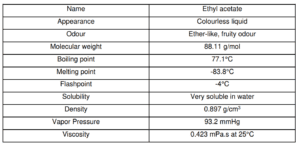
Ethyl acetate is one of the simplest carboxylate esters. An environmentally friendly organic solvent used in paint and adhesive, thus eliminating the use of aromatic compounds, in the working environment. Ethyl acetate is widely used as a solvent in chemical reactions or preparations. That’s why it is manufactured on a large scale. Ethyl acetate is also known as ethyl ethanoate.
It is also abbreviated as EtOAc. It is one organic compound and is mainly used as a solvent in different reactions. The extended formula of ethyl acetate is CH3COOCH2CH3. Ethyl acetate is highly flammable and generally used as an organic solvent in paints, films, cleaning products, etc.
Process Selection
There were various pathways and methods for producing ethyl acetate. Fischer Esterification, Direct Addition and Ethanol Dehydrogenation are some of the most considerable methods.
Fischer Esterification Process
Ethyl acetate can be manufactured by the Fisher esterification process. Fischer esterification is a form of esterification that uses an acid catalyst to reflux a carboxylic acid and an alcohol. Emil Fischer and Arthur Speier were the first to describe the reaction. The process involves treating a carboxylic acid with an alcohol in the presence of a mineral inorganic acid catalyst to produce esters.
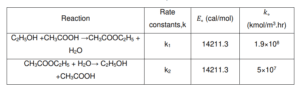
During the Fischer-Speier esterification production of CH3COOC2H5, organic compounds and acids that were used as the raw material were C2H5OH and CH3COOH. The process was utilized under the action of catalysts H₂SO₄ and CH3C6H4SO3H. Throughout the reaction, CH3COOH is representative of molecules class addressed as fatty acids and will be converted to ethyl acetate. In addition, the difference in boiling points makes it simple to separate the raw materials of ethyl acetate and acetic acid. It was proven by the azeotropic compositions found in the ternary plots.
the chemical reactions are reversible, with reaction rate constants k1 and k2 for forward and backward reactions (Table 2), respectively. To drive the equilibrium to create more ester, excess alcohol is added, following Le Chatelier’s Principle. Excess alcohol was functioning to aid the nucleophilic attack of the alcohol on the carboxylic acid’s carbonyl carbon.
Ethanol Dehydrogenation
Ethanol dehydrogenation is an appealing production process because it is easy, non-corrosive, and less hazardous, and it only requires one ethanol feedstock. The procedure can then be designed with a simple reactor, and fermented ethanol can even be used as a feedstock. Ethanol dehydrogenation also has been the subject of several ground-breaking studies Many patents on the conversion of ethanol to ethyl. acetate have been published, documenting the use of various catalysts and related operating parameters.
For example, an industrial procedure for generating ethyl acetate from ethanol has recently been established using a copper chromite catalyst There are two types of ethanol dehydrogenation processes which are oxidative and non-oxidative.
Main Occurring Equilibrium Reaction:
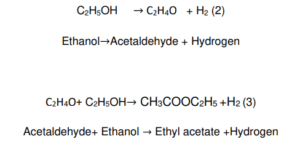
Ethyl acetate is the result of two consecutive reactions (Eq. 2,3), with the intermediate production of acetaldehyde. A rapid rate of reaction (Eq. 3) that depletes the acetaldehyde concentration favours a high selectivity for ethyl acetate. This is a crucial aspect because acetaldehyde can give place, via aldolic condensation, to several potential by-products that reduce selectivity and promote catalytic deactivation by fouling. developed a plausible full reaction network.
It is possible to obtain ethyl acetate with a satisfactory conversion by using a copper and copper chromite-based catalyst. The catalyst achieved a conversion rate of 65 % and a selectivity of 98–99 % for ethyl acetate. A copper/copper chromite catalyst contains practical support, such as alumina, and different promoters, with the main scope of preventing metal sintering and subsequent catalyst deactivation.
In line with current literature, the operative conditions are critical for obtaining high activities and selectivity. Acetaldehyde is the main reaction product at low pressure (1–5 bars). but when the pressure rises to 20–30 bars, the selectivity shifts toward the formation of ethyl acetate as the main result. Therefore, the selectivity could be reduced by the presence of a competitive reaction pathway originated by the acetaldehyde auto-condensation while operating in conditions favourable to ethyl acetate formation (Santacesaria et al., 2012).
Direct Addition of Acetic Acid to Ethylene
The direct addition of acetic acid to ethylene to make ethyl acetate has attracted much interest because it produces no by-products. As direct addition is a high atom efficiency production process, any heterogeneous catalysts with high activity and selectivity can be discovered (Inui et al., 2002b; Yamamoto et al., 2008). H4SiW12O40/SiO2 was the catalyst used in the Direct addition of acetic acid to the ethylene process. Patent literature of Showa Denko K.K. cited that SiO2- supported heteropoly acids like H4SiW12O40. are active and selective catalysts for the direct addition of acetic acid to ethylene. H4SiW12O40/SiO2 was made using an aqueous solution of H4SiW12O40 and the incipient-wetness method. In the direct addition of acetic acid to ethylene over H4SiW12O40/SiO2, ethyl acetate was selectively formed.
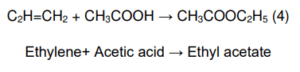
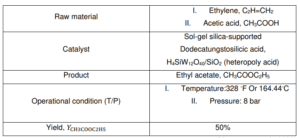
BFD of the Direct Addition Process
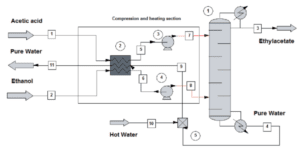
Reactor, the gas-phase reaction to produce ethyl acetate from acetic acid and ethylene was carried out at 8 bar and 328.73 ◦F. The pelletised catalysts were fixed in the reactor and treated for 0.5 hours with a gas mixture of acetic acid (37%). water (21%), and N2 (42%), flowing at a total rate of 60 dm3 h-1 (STD) at 164.85 °C. The reactant gas mixture [acetic acid (8%), ethylene (78.5%), water (4.5%), and nitrogen (9%)] was delivered into the reactor at a total flow rate of 60 dm3 h-1 after the temperature was raised to 164.85 °C (STD).
Ethyl acetate, ethanol, diethyl ether, and acetic acid were collected at the reactor’s outflow in a trap that was cooled to roughly 44.33 F for 1– 2 hours an FID-GC (Shimadzu GC-14B). (Gas Chromatography/Flame Ionization Detector) fitted with a capillary column (TCWAX, 0.25 mm 30 m). was used to quantify the amounts of each trapped product, and a Karl Fischer titration was used to measure the quantity of trapped water. The products (ethyl acetate, ethanol, and diethyl ether). that slipped through the sample trap were extracted with a syringe regularly and analysed using an FID-GC (Shimadzu GC-14B) with a glass column (Span80, 3 mm X 2 m).
Process Flow Diagram
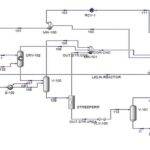
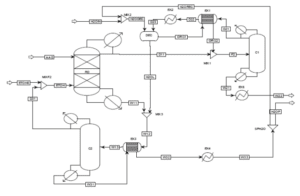
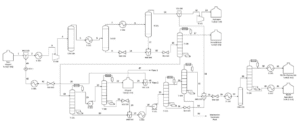
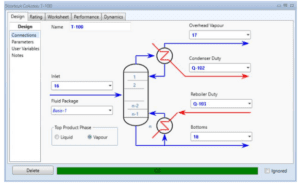
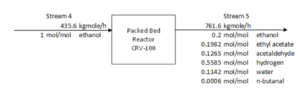
A single stream of ethanol is fed to the mixer in the chosen configuration (MIX100). The mass fraction inlet feed streams of MIX-100 are stream 1 (pure ethanol). which is composed of 100 percent ethanol, and stream 44 (recycle ethanol), which contains 99.9961 percent ethanol, 0.0032 percent ethyl acetate, 0.00005 percent acetaldehyde, and 0.00002 percent water. At the end of the mixing operation, it is believed that ethanol, ethyl acetate, acetaldehyde. and water will exit the mixer in outlet stream 2. Ethanol feed stream 2 will be heated to 100°C in the feed pre-heater. Following that, the liquid feed will enter the conversion reactor (CRV-100).
The nucleophilic addition of ethanol to acetaldehyde produces ethyl acetate and hydrogen gas in the second step. Self-aldol addition, dehydration, and hydrogenation can all be used to make aldehyde and ketone by-products from acetaldehyde. Before being fed into this CRV-100, Stream 2 from the MIX-100 is heated and compressed to a reactor operating temperature of 200-260°C and pressure of 20 bars (Carotenuto et al., 2013). This reaction produces the target product, ethyl acetate, as well as a side product, n-butanal. The reactor’s outlet stream (CRV-100), stream 5, will be cooled to 20°C in the product cooler (E-101). The utility utilised to chill this stream is 16°C
cooling water. This cooling procedure is necessary to liquefy the components other than hydrogen so that they can be separated later in the phase separator.
Continue the Description of the Process Flow Diagram
After successfully liquifying components other than hydrogen in stream 6, the hydrogen component was separated as the top product using a separator (V-100). The remaining components, such as ethanol, ethyl acetate, acetaldehyde, water, and nbutanal, will separate as liquids in the bottoms. The top outlet of V-100, stream 7, still contains acetaldehyde and traces of ethyl acetate.
As a result, this stream is cooled further to -150°C to allow the acetaldehyde and ethyl acetate components to liquify. The utility employed to chill this stream is a methane refrigerant. Returning to the phase separator (V-100) bottom output, stream 8 will enter VLV-100 to drop its pressure from 18.65 bar to 17.8 bar.
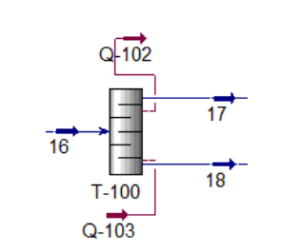
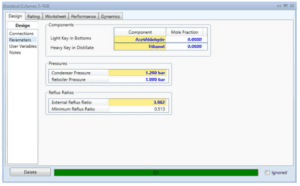
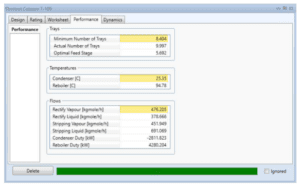
Because stream 14 will enter the mixer with stream 11 at a pressure of 17.8 bar, the pressure must be decreased. At a constant pressure of 17.8 bar, MIX-101 will mix the inlet of streams 11 and 14 with the exit of stream 15. Stream 15 from the mixer (MIX-101) will enter VLV-100 to reduce its pressure from 17.8 bar to 2.2 bar. Because the distillation column (T-100) will be operated at low pressure, the pressure must be decreased. Stream 16 will be channelled through the distillation column (T-100) to isolate acetaldehyde at the distillate. The bottom light key was set to 0.00001 acetaldehyde, while the heavy key in distillate was set to 0.00001 ethanol. The minimal reflux ratio required to meet this requirement is 0.513. Stream 17 will be held in an acetaldehyde tank before being sold to the customer with a 99.53 percent mass fraction of acetaldehyde.
Process Details
Based on the screening and scoring done from the process selection, the selected process is the production of ethyl acetate via dehydrogenation of ethanol. The process is a two-step reaction that utilizes ethanol as the initial feedstock. The first step involves a partial dehydrogenation of ethanol to acetaldehyde over a copper catalyst at elevated pressures and elevated temperatures below 250°C. For the second step, acetaldehyde will undergo a process which is the nucleophilic addition of ethanol.
Ethyl Acetate Market
End-use industries are driving the ethyl acetate market. The ethyl-acetate market is developing because of the rising demand for paints, coatings, and adhesives. The rapidly expanding construction industries, particularly in emerging nations like China, India, and Latin America, drive demand for these products. Thus, ethyl acetate’s market is being bolstered by the increased need for these items. There are a variety of reasons why ethyl acetate is used in perfumery. Powder, essences, and fragrances can all benefit from ethyl acetate’s gelling properties of ethyl acetate. Since ethyl acetate evaporates quickly on the skin, its aroma can cling to the skin and linger without leaving any undesirable residue, such as oily or alcoholic residue. In addition, ethyl acetate’s pleasant, fruity scent might contribute to the perfume’s aroma. Mascara and teeth whitening products include ethyl acetate as a component.
the selection of site location for the construction of a plant is very crucial because it can have a significant impact on the success of the industrial venture.
The Calculated Energy Balance for The Production of Ethyl Acetate
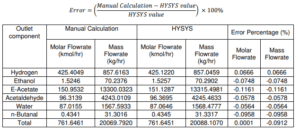
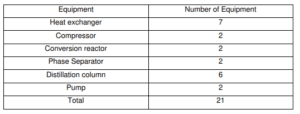
In conclusion, the calculated energy balance for the production of ethyl acetate is used to determine the cost of operating the plant to produce 100,000 tonnes per year, i.e. the energy balance on a reactor enables the determination of how much heating or cooling is required for the reactor to operate under the desired conditions. The energy balance calculation for ethyl acetate synthesis involves 21 pieces of equipment, including a heat exchanger, compressor, conversion reactor, phase separator, distillation column, and pump. Rnergy balance was estimated using numerous assumptions based on the energy conservation rule.
The total heat duty generated from the plant which represents the production of ethyl acetate via ethanol dehydrogenation is a compilation of exothermic and endothermic process which release the heat to the surroundings and also absorbs heat from surrounding respectively. The large internal energy and enthalpy changes commonly associated with chemical reactions If a reaction is endothermic, the energy were absorbs via heat from surrounding to keep the reactor temperature.
While for the exothermic, the energy was release via the heat to the surroundings The plug flow reactor, the front and rear end heaters, the compressor, and the distillation column are endothermic reactions because they absorb heat during operation. Because the values are negative, cooler is an exothermic reaction that releases heat into the atmosphere. From all the utilised data, it can be stated that there was a considerable difference between manual computation and ASPEN HYSYS calculation. Consequently, the ASPEN HYSYS and the manual .calculation most likely utilised slightly different heat capacity values
Process Simulation
Process simulation is the simulation of the process operation of a real-world process over time to explore the behaviour of the process and assess the viability of the process based on the userspecified parameters .
Chemical Process Simulation Software (CPSS) is one of the most significant simulation tools, given that the design and optimization of chemical processes require extensive calculation(Cai et al., 2017). There were several CPSS on the market, such as Aspen PLUS, Aspen HYSYS, CHEMCAD, ProMax, DWSIM, EMSO, indices Plus®, PRO/II, PetroSim, and ProCAMD. The adoption of these technologies provides advantages to manufacturing companies since the optimization of the process is performed virtually and is more cost-effective than executing the real thing.
Aspen HYSYS is one of the most prominent process simulations on the market. This software ensures that safety, output, and profits are maximised by optimising the entire site in a single environment with simulation accuracy and time-saving operations(Aspen HYSYS | Process Simulation Software | AspenTech, n.d.). It is a comprehensive modelling system used to simulate chemical processes mathematically.
Aspen HYSYS can also do chemical engineering calculations such as mass balance, energy balance, vapour-liquid equilibrium, heat transfer, mass transfer, chemical kinetics, fractionation, and pressure drop. In addition, this software includes more than 30 thermodynamic models, such as Peng – Robinson, Soave – Redlich – Kwong, NRTL, and UNIQUAC, enabling the modelling of a wide variety of processes (Thermodynamic Models & Physical Properties, n.d.). Therefore, the plant has decided to use Aspen HYSYS V11 to model the ethyl acetate production plant to assess the viability of the ethyl acetate plant. The choice of physical property methods can significantly impact the simulation’s forecast. Consequently, it is essential to use an appropriate thermodynamic method to provide an accurate simulation of the plant.
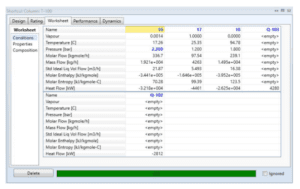
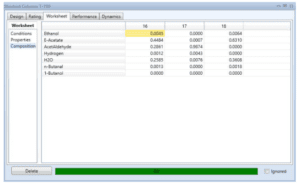
The simulation results of one of the distillation towers are mentioned below:
Distillation Column (T-100)
Chemical Components
These are the components involved in the process of manufacturing ethyl acetate. The information for each component depending on the type, class, and polarity as determined by the simulation using Aspen HYSYS. Table shows the list of components used in the process simulation.



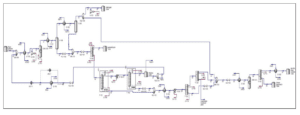
Hysys Simulation Process Flow Diagram
Process Description
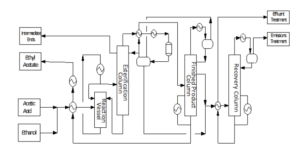
The plant was designed to produce 100,000 tonnes production of ethyl acetate with high purity of up to 99.35%. The process involved three reactions in the conversion reactor which is the first one is the partial dehydrogenation of ethanol to acetaldehyde over a copper catalyst where this process takes place in a vapour phase with the rate of ethanol conversion being around 65-70% with a selectivity of 98–99% in the optimal conditions of 200 – 250°C and 20 bar. Next, the second reaction is the nucleophilic addition of ethanol to acetaldehyde producing ethyl acetate and hydrogen gas.
This reaction occurs after the formation of acetaldehyde from the first reaction and the ethanol conversion for this reaction is around 34.65%. Then, for the third reaction which is the side reaction of self-aldol addition of acetaldehyde, followed by dehydration, and then hydrogenation to form aldehyde and ketone by-products. The major side product that was produced is n-butanal. The key steps involved in the production of ethyl acetate in this process are the reaction, followed by the separation process.
Pure ethanol is supplied in the liquid phase at 25°C with a pressure of 1 bar fed into the heat exchanger (E-100) and compressor (K-100) to increase the temperature and pressure to 245.3°C and 20 bar to achieve the optimal reaction condition. Based on the HYSYS process flow diagram, ethanol stream with a mass flow rate of 20,000 kg/hr and recycle ethanol stream with a mass flow rate of 69.91 kg/hr are mixed in MIX-100 and then heated in E-100.
Continue the Description of the Process
The outlet then will flow into the gas compressor (K100) and pressurized from 1.65 bar to 20 bar. After achieving the optimal condition for reactor condition the stream flows into the conversion reactor (CRV-100) for the reaction process. The outlet stream of the conversion reactor (stream 5) consisted of 20,069 kg/hr of vapour mixture which is then cooled using the heat exchanger (E-101) to 20°C. This step is crucial for liquifying the main product for the separation process in the phase separator (V-100). Top product of the phase separator mainly containing hydrogen gas is further cooled to -150°C and then separate using the phase separator (V-101) again until reaching total purity of hydrogen gas. The bottom outlet of the phase separator (V100) and (V-101) was mixed in a mixer (MIX-101) and further separated in a distillation column
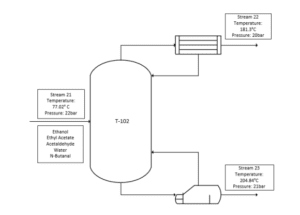
The mixer outlet, stream 15 is then depressurised to 2.2 bar for low-pressure separation of acetaldehyde at distillate in the distillation column (T-100). Distillate product manages to achieve a purity of 99.53% acetaldehyde which is stored in an acetaldehyde tank. Bottom product (stream 18) of distillation column (T-100) then flows in pressure swing distillation column (T-101) and (T-102) to remove ethanol from a 308 stream at distillate of (T-102). This method is needed to separate the azeotropic mixture of ethanol and water. Ethanol and water are azeotropes because they have the same boiling point. The feed for the second distillation column will need to be pressurized to 22 bars for further separation.
Continue the Description of the Process
The distillate of (T-102) which is stream 22 contains 98.09% of ethanol which will be recycled as feed for the process. Next, both bottom outlet of the distillation column stream 20 and 26 will be mixed in a mixer (MIX-102) and cooled down to 77.09°C for converting all the vapour traces instream into liquid. This is crucial because the outlet of the cooler (stream 28) will then pressurize in the pump (P-101) to 22 bars. The separation of water from ethyl acetate favours a high-pressure distillation column. The water separates at bottom of the distillation column (T-104) with a purity of 98.71% water and is then sent to a wastewater treatment plant for further treatment.
Then, the next process is the hydrogenation of nbutanal to 1-butanol in a hydrogenation reactor. This step is important to eliminate the azeotropic properties between n-butanal and ethyl acetate. Before the ongoing hydrogenation process outlet stream of the distillation column which is stream 31 and stream 32 will be mixed with the ratio of 1 mol of hydrogen gas to 1 mol of n butanal in a mixer (MIX-103). Then, the outlet mixer stream (stream 35) will depressurize to 4 bar and heat up to reaction temperature which is 150°C in a heat exchanger (E-104). Stream 37 will enter the hydrogenation reactor and the conversion of n-butanal to 1-butanol for about 99% will follow.
Continue the Description of the Process
Lastly, the final separation process which is the distillation column (T-105) was used to separate ethyl acetate from 1-butanol. The main product which is ethyl acetate will separate at the top column as distillate and 1-butanol will come out as the bottom product. The side product 1-butanol will then be stored in a waste by-product tank before sending to an incinerator for treatment. Meanwhile, ethyl acetate in stream 39 will be cooled to 25°C in a heat exchanger (E-105) and stored in an ethyl acetate tank. The product manages to achieve a purity of 99.35% of ethyl acetate with a total flow rate of 13,379 kg/hr. Thus, the objective of this plant to produce 100,000 tonnes of high purity ethyl acetate is achieved.
Conceptual Design
Coagulation Tank
Assumption for Coagulation Tank
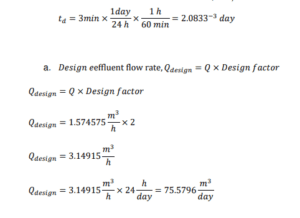
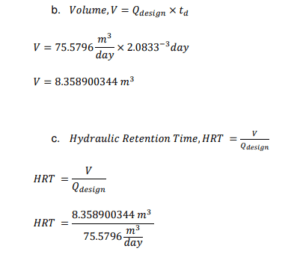
I. Design factor to cater any increase in effluent flowrate is 2
II. Proper contact time in the rapid-mix chamber is typically 3 min. (Coagulation and Flocculation & Process Fundamentals, n.d.)
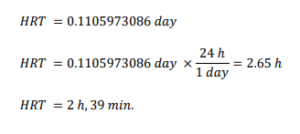
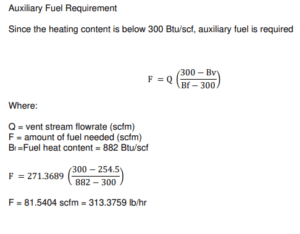
Flare Tower


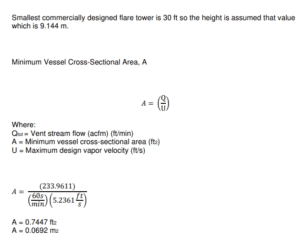


Major Equipment Design
In this project, the design of the main equipment has also been examined:
Distillation Column (T-102)
In order to concentrate the more volatile liquid in the first fractions and the less volatile components in the later fractions, a distillation column is a tube that offers surfaces on which condensations and vaporisations can occur before the gas hits the condenser. As a result, the features of liquid mixtures’ vapour pressure affect distillation processes. Vapour pressure is created by using heat as a separating agent. The new phases in the distillation have a different heat content than the first phases. The substance that emerges from the top of the column is referred to as distillate or above. High volatile, low boiling point, or light constituents may condense as vapour or evaporate as distillate. Condensate, the bottom product, will emerge from the column in the interim. Less volatile, highly flammable, or heavier constituents may concentrate and emerge from the distillation column as condensate.
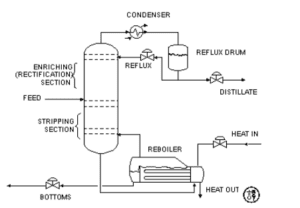
Distillation column is used in the process in order to strip ethanol from the mixture components of ethyl acetate, acetaldehyde, water and n-butanal. T-102 is a continuous column where the feed continuously fed into the column at 77.02°C. Stream 20 consists of ethanol, ethyl acetate, acetaldehyde, water and n-butanal are fed into column with pressure of 22bar. The top product is ethanol only as the after equipment unit is ethanol storage tank, whereas the desired product is at bottom stream. These components are considered as ideal solution.
PROCESS CONTROL
Automatic process control aims to keep process variables like temperatures. pressures, flows, compositions, and the like at a desired operating value under controlled circumstances because processes in nature are constantly changing. If appropriate actions are not taken to respond, the crucial process parameters related to safety, product quality, and production levels fail to achieve their design conditions. Process control is one of the most crucial components of every industrial facility. Monitoring changes in operating circumstances, compositions, and the physical characteristics of the streams are the most crucial components of the operations.
The system will be automated after the creation of process controls. By detecting the output parameter’s amplitude, comparing it to a predetermined or desired level, and feeding a feedback signal to regulate an input variable, process control automatically controls an output variable (Areej, 2018).
Continue to Control the Process
The four categories of variables that are essential in the process control system are controlled variables, manipulated variables, disturbance and set point. The value of the controlled variable must be maintained constant or restricted to a certain range. On the other hand, the controlled variable is maintained at its set point using the manipulated variable. The set point is the desired value of the controlled variable at that precise moment in time.
The set point is the ideal value for an output parameter or variable that a sensor is tracking. and it is referred to as a system where a warning message will be sent if the value deviates from this. Therefore, the primary responsibility of the control system is to maintain the set point by modifying the controlled variable to correct the set point’s incorrect value.
To ensure that the plant can be operated safely during the manufacturing of ethyl acetate, it is crucial to have a good control system inside the facility. To avoid any risk to the people or the property. it is necessary to continuously monitor all the operation’s parameters. Following safety procedures is necessary for the plant to run efficiently,
The commercial Production of Ethyl Acetate
The commercial production of ethyl acetate is primarily accomplished through two processes: the Tishchenko reaction, which produces ethyl acetate by direct conversion of ethanol via acetaldehyde using an aluminium alkoxide catalyst. and the direct esterification of ethanol with acetic acid using a sulphuric acid catalyst.
Even though this process was commercialized. it still has an issue where for esterification process needs to be manufactured from high alloyed steel due to the high corrosivity of acetic and sulfuric acids. Thus, we have selected a well-known industrial route for synthesizing ethyl acetate via the dehydrogenative dimerization of ethanol. There are two ways this can be done, oxidative and non-oxidative:
-
Oxidative – The oxidative dehydrogenative dimerization of ethanol is as follows:

2. Non-oxidative – The non-oxidative dehydrogenative dimerization of ethanol is as follows:
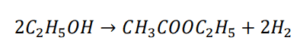
This is the main route for dehydrogenative dimerization of ethanol in industry. The hydrogen in ethanol is removed to produce acetaldehyde. which is then added to ethanol to form hemiacetal, which is subsequently dehydrogenated to give ethyl acetate.
As a result, non-oxidative hydrogenation dimerization of ethanol was chosen as the production process of ethyl acetate. It is cheaper and more environmentally friendly because of ethanol.
Mass Balance of Equipment
This section shows all the mass balances at each piece of equipment, as well as their manual calculations to obtain the required quantity of production rate. Based on this value, it should be able to compute the amount of raw material needed to generate the desired final output. Atomic and molecular balancing is the manual calculation method for this system. There may be some errors when comparing manual Excel calculations to Aspen HYSYS simulation.
Regarding Mass Balance, one of the equipments is mentioned below:
Conversion Reactor:
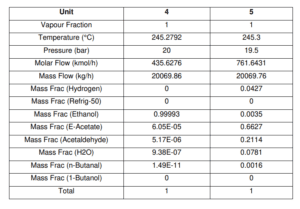
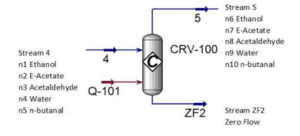
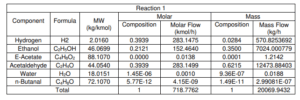
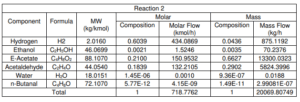
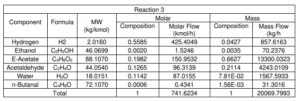
Figure shows the schematic diagram of the conversion reactor (CRV-100) inlet and outlet component streams which are Stream 4, Stream 5 and Stream ZF2 respectively. In this reactor, the first front-end reaction process is partial dehydrogenation of ethanol to acetaldehyde over a copper catalyst. For the second reaction is nucleophilic addition of ethanol to acetaldehyde producing ethyl acetate and hydrogen gas. acetaldehyde can undergo self-aldol addition, followed by dehydration, and then hydrogenation to form aldehyde and ketone by-products. The major side product that we focused on was n-butanal.
Stream 2 from MIX-100 is heated and pressurized to reactor operating temperature about 200-260°C and pressure 20 bars before being fed into thisCRV-100 (Carotenuto, Tesser, di Serio, et al., 2013). The desired product, ethyl acetate is achieved from this reaction and side product which is n-butanal was formed. Extent of reaction method is used to perform the material balance calculation of a reactive system (Felder & Rousseau, 2014c). Extent of Reaction method is introduced in CRV-100 since the equipment is involves with process and generate a new product. The method used to calculate which reaction is reacted and unreacted.
Main Reaction
i. C2H5OH → CH3COH + H2
ii. CH3COH + C2H5OH → CH3COOCH2CH3 + H2
Side Reaction
i. 41.346CH3COH + 10H2 → 0.5C4H8O + 100.224H2O
Assumptions made for this process unit are stated as follows:
I. Mass in is equal to mass out.
II. CRV-100 is a steady-state reactor.
III. CRV-100 is an isothermal plug flow reactor
IV. No accumulation occurred in the reactor.
V. Conversion of ethanol for first reaction is 65%
VI. Conversion of ethanol for second reaction is 34.65%
VII. Conversion of acetaldehyde for side reaction is 0.2%
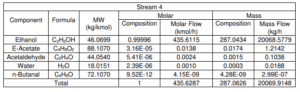
For Reaction 1 (C2H5OH → CH3COH + H2)
Based on stoichiometric reaction, 1 mol of ethanol is to produce 1 mol of acetaldehyde and hydrogen gas.
1 mol ethanol: 1 mol acetaldehyde
Conversion for reaction 1 = 65%
Ethanol feed rate = 435.6115 kmol/h
Converted ethanol = 0.65 × 435.6115 kmol/h = 283.1475 kmol/hr
1 mol Acetaldehyde produced = 1 mol H2 produced = 1 mol Ethanol reacted = 283.1475 kmol/hr
Ethanol remains = 435.6115 – 283.1475 = 152.4640
Energy Balance on Each Equipment
Energy balance calculation will involve according to equipment list in table below:
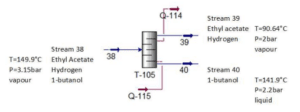
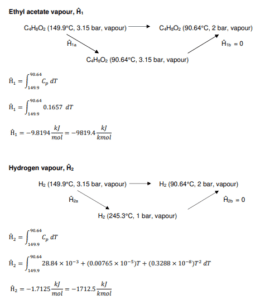
As an example, we refer to the Distillation column device:
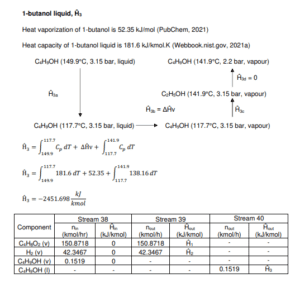
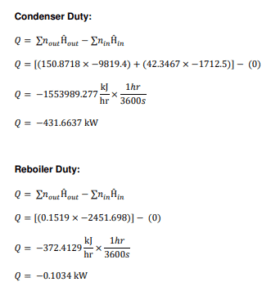
T-105
Figure shows the methanol distillation unit (T-105) which involves a non-reactive process. The purpose of this unit is to remove 1-butanol from the inlet Stream 38. The 1- butanol waste stream leaves the distillation tower through bottom stream as wastewater while ethyl acetate, our main product leaves through the top of the tower.

Economic Design
Economic analysis is critical in designing plants. A break-even analysis is necessary for a chemical plant to evaluate profit and offer information on the amount of investment required. Economic analysis is the study of the capital costs, equipment costs, raw material costs, labour costs, and working capital associated with the construction and operation of chemical processes. Fixed capital is the cost of a fully operational facility, whereas working capital is the additional expenditure required for plant design.
In this project, the following items have been analyzed, some of which have been mentioned:
Raw Material Cost Estimation، Capital Cost Estimation، Capital Investment، Operating Labour Cost Estimation، Manufacturing Cost Estimation، Utility Cost Estimation، Waste Treatment Cost Estimation، Variable Cost Estimation، Total Fixed Cost Estimation، Total Revenue Estimation، Break-Even Analysis، Profitability Analysis
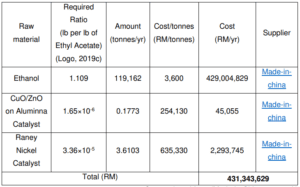
Raw Material Cost Estimation
Capital Cost Estimation
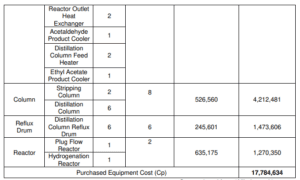
There are five broad classes of capital cost estimates that are frequently used in the process industries which are detailed estimate, definitive estimate, preliminary estimate, study estimate and order-of-magnitude estimate. In study estimate makes use of a list of the process’s primary equipment. This category contains all pumps, compressors, turbines, as well as columns and vessels, as well as fired heaters and exchangers. Each piece of equipment is approximated in size and cost. After factoring in the overall cost of equipment, the estimated capital cost is calculated.

Capital Investment
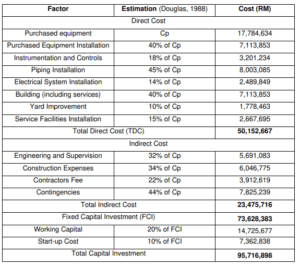
Apart from the operational and installed equipment expenses, there are various costs associated with the construction and operation of a chemical plant. Some of these costs are capital-related, while others are operating-related. Fortunately, most of these costs can be directly tied to the cost of installed equipment via the application of various factors.
Manufacturing Cost Estimation
The cost of manufacturing COM is determined using the following equation.
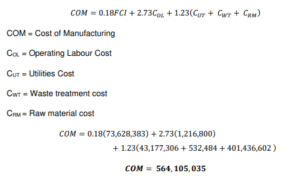
Variable Cost Estimation
Variable Cost = Raw Material Cost (CRM) + Labour Cost (COL) + Utilities (CUL) + Land Cost
The land price at Sungai Ara, Bayan Lepas, Penang for 1 acre of land, according
to Property Guru, is RM2,613,600/acre. Thus, the total land cost is:


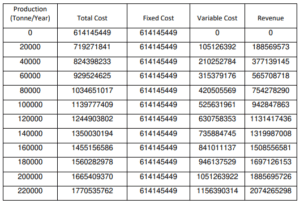
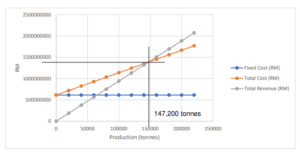
Break-Even Analysis
The break-even analysis aims to identify the revenue required to cover the whole cost of construction of the plant, including fixed and variable costs. When total revenue equals total expense, the Breakeven Point is reached.

Plant layout design-3-Dimensional




Conclusion
Various process intensification techniques have been carried out to produce ethyl acetate. including the Fischer process of esterification of acetic acid with ethanol. Dehydrogenation of ethanol to ethyl acetate and direct addition of acetic acid to ethylene. The influence of various process parameters, such as product yield, process conditions, raw materials, process safety issues were also investigated.
Ethanol dehydrogenation process is the most suitable pick for producing ethyl acetate. Could be concluded that the process was preferable for its four properties which were availability of raw material. conversion and product yield, operating cost, and profit margin. Due to the relatively expensive cost of raw ingredients and the risk of undesired by-products emerging via alternate synthesis pathways. the conventional Fischer Esterification is determined to be the most utilised reaction. Fisher Esterification came in second for the process condition.
In this project, the simulation of ethyl acetate process has been done in Aspen Hysys software. And explained the process, equipment design, mass balance, economic evaluations, etc.