Introduction
With the establishment of the Ilam Gas Refinery for the purpose of refining gas from the Tang Bijar and Kaman Kooh gas fields located in Ilam province, the Ilam Petrochemical Complex plan was approved during the 947th session of the National Petrochemical Industries Company Board. The company was founded on July 6, 2003. The Ilam Petrochemical Company, covering an area of 122 hectares in the Chavar region, is located 18 kilometers northwest of Ilam City.
Process of the Ilam Petrochemical Olefin Plant
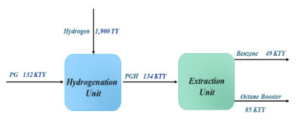
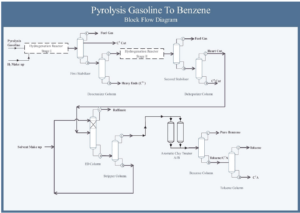
The Ilam Petrochemical Olefin Plant (ILPC) aims to extract “benzene” and “octane improver” from “pyrolysis gasoline (PG)” as benzene (PTB).
The goal of the Extractive Distillation (ED) process is to produce high-purity aromatic products. A unique feature of the Morphylane ED process is the use of N-formylmorpholine (NFM) solvent, which optimally combines selectivity and solvent efficiency. In the ED process, NFM changes the vapor pressure of the separated components. The vapor pressure of aromatic materials is lower than that of non-aromatic materials. Non-aromatic vapors are removed with a portion of the solvent from the top of the ED column, which is recovered in a smaller column that can either be installed on the main column or separately. The bottom product of the ED column is sent to a stripper unit to separate the pure aromatics from the solvent. After intense heat exchange, the solvent is recycled back to the ED column. NFM fulfills the necessary solvent properties for this process, including high selectivity, thermal stability, and suitable boiling point.
This process consists of three main units:
Unit 100: Partial Hydrogenation
If pure aromatic materials are to be obtained, the feed must be pre-treated before non-aromatic and aromatic materials are separated by extractive distillation. Catalytic hydrogenation processes have become the most suitable technology for removing impurities such as diolefins, olefins, sulfur, nitrogen, and oxygen components. Due to the high diolefin content, raw pyrolysis gasoline from steam crackers tends to polymerize and form sludge even when stored in nitrogen-covered tanks. Since polymerization is encouraged at higher temperatures, diolefins must be selectively hydrogenated at relatively low temperatures using highly active catalysts.
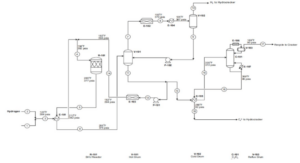
Unit 200: Complete Hydrogenation
Impurities such as sulfur, nitrogen, oxygen components, and olefins are hydrogenated in the complete hydrogenation step. The reactions occur in the gas phase on nickel/molybdenum and/or cobalt/molybdenum catalysts at reactor inlet temperatures between 240 to 320 degrees Celsius. For this purpose, thermal decomposition due to selective hydrogenation occurs through heat exchangers and a process heater after mixing with recycled hydrogen. After cooling, the reactor product is sent to a high-pressure separator where unconsumed hydrogen is separated from the liquid phase.
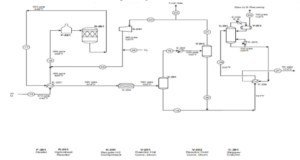
Unit 300: Extractive Distillation
In the extractive distillation (ED) process, the solvent changes the vapor pressure of the separated components. Non-aromatic vapors exit the ED column along with some solvent vapors. Internal reflux is controlled by the solvent feed temperature. The solvent is recovered from the overhead product in a small column with sectional reflux, which can either be installed on the main column or separately. The bottom product from the ED column is fed to the distillation step, the stripper, to separate pure aromatics from the solvent. After intense heat exchange, the solvent is recycled back to the ED column.
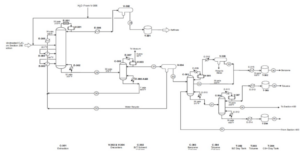
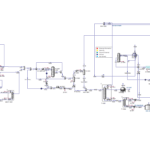
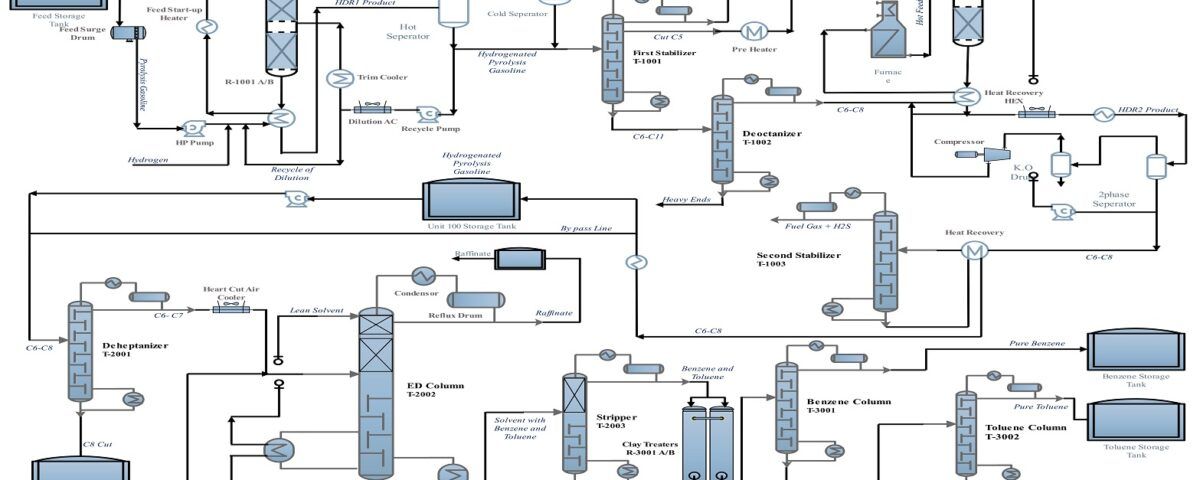
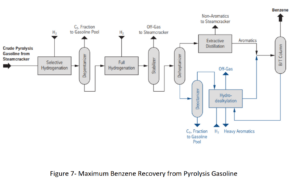
Process Flow of Converting Pyrolysis Gasoline to Benzene
The provided flow diagram graphically displays the various stages of converting pyrolysis gasoline to pure benzene. This process is of significant importance in petrochemical industries for producing benzene as a raw material for manufacturing various chemical products such as styrene, phenol, and caprolactam. The process begins with introducing pyrolysis gasoline as the feed. Pyrolysis gasoline is combined with hydrogen in two hydrogenation stages to convert its unsaturated aromatic compounds to saturated compounds. The output products from the hydrogenation reactors enter stabilizers to separate lighter gases from liquids.
In several distillation columns, various components are separated based on boiling points. The decanter, depentanizer, ED, stripper, benzene, toluene, and C9 Aromatics columns play key roles in this stage. The output product from the depentanizer enters an aromatic clay refining unit to remove impurities and discoloration. Pure benzene is the main product of this process, used in various industries for manufacturing plastics, paints, and adhesives. Toluene is an aromatic solvent used in producing paints, adhesives, and explosives. C9 Aromatics is a mixture of heavier aromatic compounds than toluene used in producing resins and plastics.
Economic Feasibility Study for Benzene and Toluene Production at Ilam Petrochemical for Petrol Company
Ilam Petrochemical will play a vital role in producing benzene and toluene, two aromatic hydrocarbons with widespread industrial applications. This plan outlines the production process, essential equipment, and safety and environmental considerations required for the successful operation of benzene and toluene production.
1. Overview of Benzene and Toluene Production
Benzene (C6H6) is a vital raw material in the petrochemical industry, primarily used in the production of styrene, phenol, and cyclohexane, which are precursors for various plastics, resins, and synthetic fibers. Toluene (C7H8) is used in the production of explosives, paints, coatings, adhesives, and as a solvent in various chemical processes.
2. Production Process
The production of benzene and toluene typically involves catalytic reforming of naphtha, a hydrocarbon mixture derived from crude oil. The steps of this process are described below:
Step 1: Naphtha Feed Preparation
Naphtha feed is desulfurized to remove sulfur compounds that could poison the catalysts used in later stages. The desulfurized naphtha is then fractionated to achieve the desired boiling range for reforming.
Step 2: Catalytic Reforming
The prepared naphtha enters a series of reactors containing platinum-based catalysts. The conditions in the reactors (high temperature and pressure) promote the formation of aromatics (benzene, toluene, and xylene) through dehydrogenation and isomerization reactions.
Step 3: Aromatic Extraction
The reformate undergoes extraction to separate aromatic hydrocarbons (benzene, toluene, and xylene) from non-aromatics. Common extraction methods include liquid-liquid extraction and extractive distillation. The aromatic-rich stream is further separated into individual product streams of benzene, toluene, and xylene.
Step 4: Purification
Each aromatic product undergoes further distillation to achieve the purity specifications required for commercial use.
Our proposed economic plan has been updated following thorough reviews and comparisons with the successful model of the Persian Gulf Holding, utilizing the latest scientific and engineering achievements, ready for the construction and commissioning phase (EPC). Collaborating with a reputable technical and engineering company ensures the success of this project.
APIPCO, relying on deep technical knowledge, is capable of providing comprehensive solutions for benzene extraction from pyrolysis gasoline. The company has successfully executed several projects in this area, including:
1. Providing aromatic technical knowledge to Marun Petrochemical.
2. Delivering COMFAR and pre-feasibility studies.
3. Providing initial technical knowledge and feasibility study for the project implementation for Ilam Petrochemical and Petrol Company.