Introduction
In today’s world, the production of base chemicals and their derivatives is of paramount importance across various industries. The processes for producing methanol, dimethyl ether (DME), propylene, and polypropylene play a vital role in meeting industrial demands, finding applications in sectors such as automotive, textiles, and many others.
This project examines the production processes of methanol, dimethyl ether (DME), propylene, and polypropylene (specifically, polypropylene production from natural gas). These chemicals are particularly significant due to their extensive applications in diverse industries. The simulation of these processes was conducted using engineering software like Aspen Plus and Aspen HYSYS to accurately evaluate their efficiency, energy consumption, and yield. The selection of precise thermodynamic models, such as Peng-Robinson and PC-SAFT, allows for accurate prediction of material properties and the improvement of production processes.
1. Methanol Production
Methanol (CH₃OH) is an essential chemical in various industries, with wide-ranging applications including the production of various derivatives and fuels.
Description of the Methanol Production Process
The industrial method for producing methanol from natural gas consists of several key steps:
1. Desulfurization of Natural Gas: In this step, sulfur compounds are removed from natural gas to avoid poisoning the catalysts used in subsequent stages.
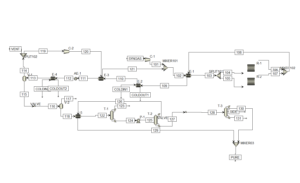
2. Synthesis Gas Production: After desulfurization, natural gas is converted into synthesis gas, typically through Steam Methane Reforming (SMR), Partial Oxidation (POX), or Autothermal Reforming (ATR).
3. Synthesis Gas Compression: The produced synthesis gas is compressed at high pressures, preparing it for the methanol synthesis stage.
4. Methanol Synthesis: In this stage, the synthesis gas is converted into methanol under controlled temperature and pressure conditions using catalysts based on copper oxides (CuO/ZnO). This process is generally exothermic and occurs at low temperatures and high pressures.
5. Methanol Distillation: The crude methanol produced from the synthesis process is subjected to distillation to remove impurities and achieve the required purity levels.
Process Simulation of Methanol Production
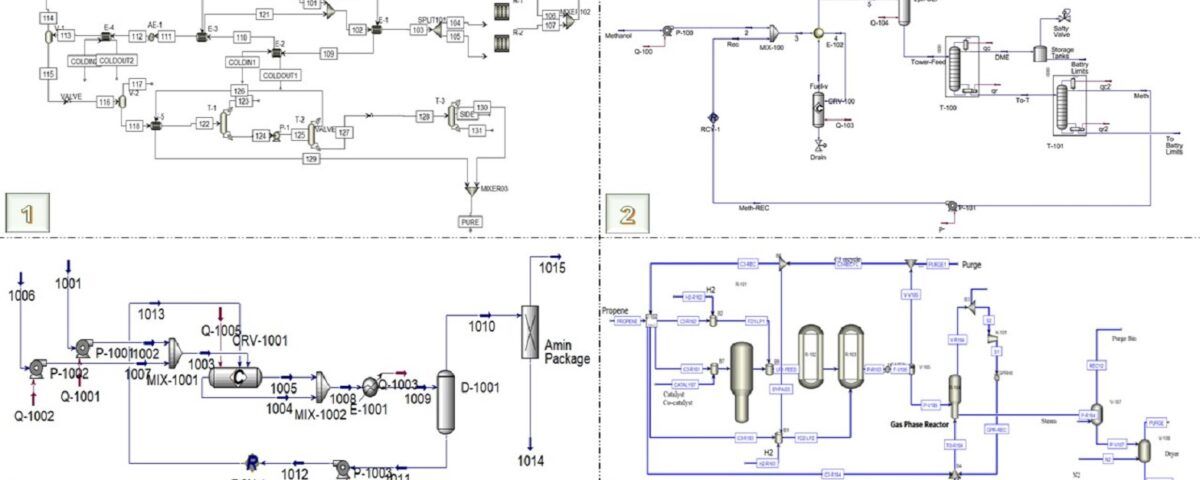
The simulation of the methanol production process was conducted using Aspen Plus V12.0 and Aspen HYSYS. The simulation utilized the Peng-Rob and PSRK thermodynamic packages, specifically suitable for modeling systems comprising synthesis gases and light materials. 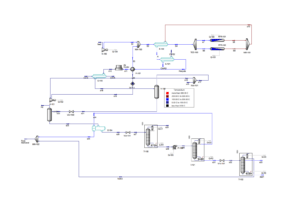
Simulation Steps
1. Synthesis Gas Compression: The incoming synthesis gas, initially composed of CO, H₂, CO₂, and minor components, enters compressor C-1 at an initial pressure of 33.2 bar and a temperature of 40°C. In this compressor, the synthesis gas is compressed to 76 bar to create the necessary conditions for the reactions in the methanol synthesis reactors.
2. Pre-heating and Entry into the Reactor: The compressed synthesis gas stream is pre-heated in heat exchanger E-1, exchanging heat with the outgoing stream from the reactors. This heat exchange increases the temperature of the synthesis gas from 111.5°C to 180°C. Pre-heating plays a significant role in enhancing reaction yield, as higher temperatures facilitate exothermic reactions.
3. Methanol Synthesis in Isothermal Reactors: The incoming stream is divided into two isothermal reactors operating at a temperature of 264°C. These reactors are designed using CuO/ZnO-based catalysts, which are widely used in methanol synthesis due to their high activity and thermal stability under high temperature and pressure conditions.
4. Cooling and Product Separation: The output stream from the reactors is combined and gradually cooled in heat exchangers E-1, E-2, E-3, AE-1, and E-4 to a temperature of 40°C. During these stages, the temperature of the flow is continuously decreased, and its thermal energy is recovered in different parts of the process.
5. Final Purification in Distillation Columns: After pre-heating in heat exchanger E-5, the crude methanol enters distillation column T-1. In this column, remaining light gases and some methanol are removed from the top product. The bottom product of column T-1, containing 83 mol% methanol and 17 mol% water, is sent to the second distillation column (T-2).
6. In column T-2, methanol with 99.9 mol% purity is obtained from the top of the column, and after cooling in heat exchanger E-5, it is collected as the final pure methanol product.
Results and Analysis
The energy consumed in compressor C-1 is 23.13 MW, and in compressor C-2, it is 10.60 MW. Additionally, methanol production achieved a purity of 99.6 weight% in the final stream, indicating the favorable performance of the simulated process. The output of this methanol production unit will be used as feedstock in the simulation of the dimethyl ether unit, which will be discussed in subsequent sections.
Production of Dimethyl Ether
Dimethyl ether (DME) is a gaseous organic compound that has gained significant attention in various industries due to its unique properties. Under standard conditions, DME exists as a gas and is used as a clean fuel and a substitute for LPG and diesel, as well as a raw material in chemical synthesis. This compound is considered a highly suitable option for various industrial and domestic applications due to its low toxicity, appropriate flammability, and biocompatibility.
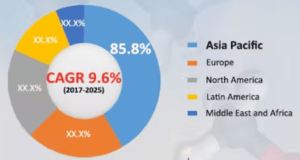
The global DME market is divided into five main regions: Asia, Europe, America, Latin America (LATAM), and the Middle East and Africa (MEA). Among these, Asia holds a major share of the global market because of extensive DME production and consumption in China. This country accounts for over 85% of global DME market share, playing a crucial role in shaping market trends. Alongside China, Japan, India, and South Korea also contribute significantly to the increased use of DME in the Asian region.
Methods for Producing Dimethyl Ether
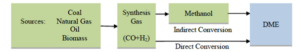
DME can be produced through several methods, but the two main and widely used approaches are direct and indirect production methods.
Indirect Method
In the indirect method, dimethyl ether is produced from methanol. First, methanol is synthesized from synthesis gas in a two-step process and then converted into DME in a separate reactor. This process involves exothermic reactions that must occur at lower temperatures to achieve optimal yield, due to the formation of by-products at higher temperatures.
Direct Method
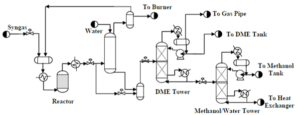
In the direct production method of DME, synthesis gas is converted into DME in a single step in the presence of bifunctional catalysts. This method requires precise temperature control, as the reactions producing DME from synthesis gas are highly exothermic. The direct synthesis (STD) process from synthesis gas has many complexities, but utilizing chemical engineering operational units such as adsorption, flash evaporation, and distillation enables the separation and purification of DME.
Simulation of the Dimethyl Ether Production Process
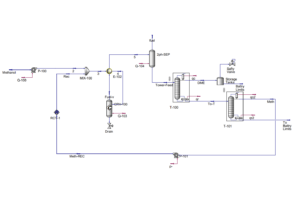
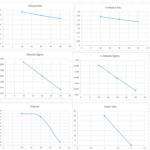
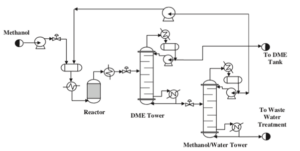
The production process of dimethyl ether through the indirect method using methanol as a feedstock has been simulated in Aspen HYSYS V12.0. In this simulation, the Uniquac thermodynamic package was applied, and the process reactor was considered as the heart of the system with an assumed 80% conversion of methanol to DME.
The process includes various stages such as increasing feed pressure, heating the incoming methanol in a heat exchanger, reacting in the reactor, separating products in distillation columns, and recycling methanol. The simulation shows that despite the existing challenges, it is possible to produce high-purity DME with satisfactory yield.
Simulation Results
The results show that at a temperature of 325°C and pressure of 64.5 bar, the reactor output includes DME, unreacted methanol, and water. After separating light gases and distilling, the stream is sent to two distillation columns to separate methanol and water from DME. Ultimately, DME is produced with a molar purity of 99.9% as the final product.
Energy Consumption
The energy consumption in the various system equipment differs significantly, where the highest energy consumption is attributed to the duty reboiler of column T-2 at 129 MW, and the lowest to pump P-101 at 0.154 MW. The DME synthesis reactor produces an output stream with a molar flow rate of 7524 kmol/h, with a molar composition of 0.3978 DME.
This simulation serves as a starting point for designing and optimizing DME production units aimed at enhancing yield and reducing energy consumption. Additionally, the output of this simulation will be utilized as input for the simulation of subsequent units, including propylene production.
Production of Propylene
Propylene, as one of the vital light alkenes, plays a significant role in the petrochemical industry and chemical production. Besides its use in polymerization to produce polypropylene, it is a key feedstock in manufacturing other valuable products such as propylene oxide, oxo alcohols, acrylonitrile, and cumene. Given the increasing demand for propylene, developing optimized methods for producing this compound is essential. Traditional methods, such as pyrolysis and catalytic cracking, which still account for the majority of global propylene production, are inadequate to meet the full demand due to various by-products generated. Therefore, innovative and optimized technologies for dedicated propylene production with higher yields are being developed.
The Propylene Production Process
Pyrolysis
Pyrolysis is one of the primary processes for propylene production, during which hydrocarbon feedstocks are decomposed under high-temperature conditions (790-900°C) and short contact times (0.1-0.5 seconds) in the presence of steam. By changing the type of feedstock and optimizing process conditions, propylene yields can be increased.
Catalytic Cracking
Catalytic cracking is another method for producing propylene, where hydrocarbon feedstocks are subjected to specific catalysts. By optimizing operational conditions and using zeolite catalysts such as ZSM-5, propylene production yield can be increased by up to 20% by weight. In recent years, these processes have been extensively developed to increase the production of light alkenes, particularly propylene as a valuable raw material.
Propane Dehydrogenation
The propane dehydrogenation process, such as Catofin and Oleflex, produces propylene by removing hydrogen from propane. Oxidative dehydrogenation is another optimized approach performed at lower temperatures, which helps reduce coke formation and extend catalyst life.
Propylene Production from Natural Gas
Producing propylene from natural gas is an innovative and promising method based on converting methane to synthesis gas and then producing alkenes from synthesis gas or its derivatives, such as methanol or dimethyl ether (DME). The route utilizing DME is recognized as a preferable option due to its lower toxicity compared to methanol and the economic viability of the process.
Simulation of the Propylene Production Unit
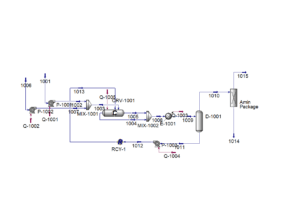
The simulation of the propylene production unit using dimethyl ether (DME) feedstock was conducted with Aspen HYSYS V12.0. In this simulation, the PSRV thermodynamic package was chosen, providing high-accuracy modeling of mixtures containing water, hydrocarbons, and various gases.
To achieve more accurate reaction modeling, a conversion reactor was used. The conversion percentage for each reaction was precisely set to align with optimal operational conditions.
Simulation Process Overview
At the start of the process, high-purity DME feedstock at a temperature of 12°C and pressure of 4 bar, along with a recycle stream predominantly containing water, reaches a pressure of 41 bar after passing through pumps P-1001 and P-1002. The combination of these streams occurs in a mixer, followed by the mixture entering reactor CRV-1001. In this reactor, the specified reactions occur at a high temperature (~400°C).
The output stream from the reactor, which includes a mixture of hydrocarbons, water, and by-products, is first cooled to 40°C in heat exchanger E-1001. It then enters the two-phase separator D-1001. In this unit, water along with a small amount of hydrocarbons is separated and returned to the reactor to assist the recycling process and enhance overall yield. After water removal, the hydrocarbon stream, free of water, is sent to an Amine Package for carbon dioxide separation.
Simulation Results
Simulation results indicate energy optimization and achieving favorable conditions for propylene production:
1. Energy Consumption: The energy required for pumping and reactor processes, along with the energy rejected in the cooler, are 3200 MW for the reactor and 3237 MW for the cooler. These values illustrate precise thermal balance and effective energy management within the system.
2. Input and Output Stream Conditions: The output stream from the reactor has a molar flow rate of 220,300 kmol per hour and a mass flow rate of 4.8 million kilograms per hour, containing 10.51% molar propylene. The final propylene product is produced at a mass flow rate of 56,000 kilograms per hour with a 52% weight purity.
3. Product Quality: The final propylene product reaching 30.63°C and 20 bar pressure presents favorable conditions for subsequent processes, such as polypropylene production.
Production of Polypropylene
Polypropylene (PP) is a semi-crystalline polymer belonging to the family of polyolefins, produced from the monomer propylene. This polymer is synthesized using catalysts and through the polymerization of the gaseous propylene monomer at room temperature. In the polymerization process, propylene molecules link together to create long polymer chains that ultimately produce solid polymers with desirable mechanical properties and extensive applications.
The development and improvement of catalysts play a crucial role in the polymerization process, as enhanced catalyst efficiency results in purer products while simplifying and reducing the cost of the production process. Polypropylene is primarily divided into three main types:
1. Homopolymers: Due to high thermal resistance and good strength, they are suitable for various applications.
2. Copolymer: Produced by combining different monomers. They are extensively used in the automotive and industrial sectors due to their high flexibility.
3. Random Copolymers: Created by adding ethylene to the polypropylene chain, enhancing optical properties such as transparency.
Polypropylene Production Processes
Polypropylene production can be achieved through two main methods: gas-phase polymerization and liquid-phase polymerization, each with specific characteristics and advantages.
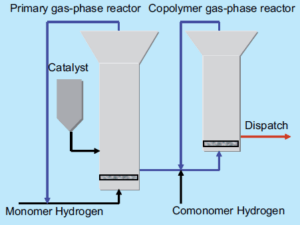
Gas-Phase Polymerization
In this process, gaseous propylene interacts with solid catalysts in a fluidized bed or stirred reactor. The fluidized bed process creates a dry polymer powder by passing propylene through a gas distributor plate, leading to rapid heat removal and high reaction efficiency. The stirred bed method employs a reactor with mixing chambers, allowing simultaneous polymerization and mixing.
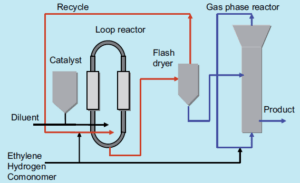
Liquid-Phase Polymerization
In this method, the catalyst and polymer are suspended in an inert solvent, usually a light or heavy hydrocarbon. Supercritical slurry polymerization processes utilize supercritical propane as a diluent. These processes typically occur in loop or stirred reactors with solvent and can employ various types of catalysts. Advanced processes often combine a loop reactor with one or two gas-phase reactors, resulting in enhanced product properties and higher efficiency.
Simulation and Results
The simulation of the polypropylene production unit was conducted using Aspen Plus V10.0. To accurately predict the behavior of materials and their physical and thermodynamic properties, the Perturbed-Chain Statistical Associating Fluid Theory (PC-SAFT) thermodynamic model was utilized. This model is particularly suitable for simulating the behavior of polymers and complex solutions, providing high accuracy in predicting properties of various phases and their equilibrium.
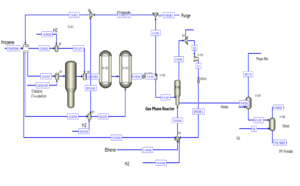
Simulation Process Description
In this simulation, propylene gas (C₃H₆) serves as the main feedstock. The propylene gas, after mixing with unreacted propylene from previous stages along with the catalyst and co-catalyst, enters the first polypropylene production reactor designated as R-101.
Reactor R-101 operates at 20°C and 35.5 bar pressure. The output from this reactor consists of a mixture of polypropylene and unreacted monomers. This stream then mixes with fresh propylene and hydrogen, entering two subsequent liquid-phase reactors (R-102 and R-103). These reactors operate under similar conditions to the first reactor, gradually increasing the conversion percentage of propylene to polypropylene.
The output from the third reactor, after passing through a two-phase separator that removes unreacted gases (mainly propylene) from the liquid phase, enters a gas-phase reactor. This reactor, designed for completing polymerization reactions, operates such that the incoming liquid stream, primarily consisting of polypropylene, undergoes final reactions at 75°C and 15.84 bar pressure, producing high-purity polymer. The final output, after drying and removing non-polymeric materials, is obtained as pure polypropylene.
Simulation Results Analysis
Overall, this simulation demonstrates that by precisely selecting operational parameters like temperature, pressure, and reactant ratios, high efficiency in propylene production can be achieved. The use of advanced thermodynamic models like PC-SAFT enhances the accuracy of simulations, allowing for more precise predictions of complex system behaviors. This information enables process engineers to optimize the design and operation of industrial units and reduce production costs.
Conclusion
The simulation and analysis of the production processes for methanol, dimethyl ether, propylene, and polypropylene indicate that by carefully selecting operational conditions and suitable thermodynamic models, optimal and high-quality production can be achieved. Each of these processes presents specific challenges and complexities; however, through advanced technologies and chemical engineering knowledge, these challenges can be effectively managed and improved.
The results from these simulations demonstrate that optimizing operational parameters not only reduces production costs but also contributes to minimizing environmental impacts. This project has proven that combining specialized knowledge with sophisticated simulation tools enables achieving sustainable and economical industrial production.
Simulation of Continuous Processes for Polypropylene Production from Natural Gas
In this project, the simulation of continuous processes for polypropylene production from natural gas has been carried out using Aspen Plus and ASPEN HYSYS software. This project includes a report to facilitate a better understanding of the simulation process and the analysis of results.