Introduction
Formaldehyde is a simple organic compound with the chemical formula H₂CO. It is a colorless gas with a pungent odor. Formaldehyde is widely used as a preservative, disinfectant, and adhesive in various industries.
Today, the production of formaldehyde as a high-demand chemical product in various industrial, medical, and agricultural sectors has gained significant attention. Large-scale production of formaldehyde is achieved through the catalytic conversion of methanol to formaldehyde via two processes: catalytic dehydrogenation of methanol to formaldehyde and catalytic oxidation of methanol to formaldehyde. The industrial process of methanol oxidation to formaldehyde is more preferred due to its simpler process design and lower cost.
Properties of Formaldehyde
- Colorless gas soluble in water: Formaldehyde readily dissolves in water, and the resulting aqueous solution is called formalin.
- Pungent odor: It has a distinctive pungent odor that can be irritating to the eyes, nose, and throat at high concentrations.
- Toxicity: Formaldehyde is a toxic substance, and long-term inhalation or direct contact can lead to health problems such as skin and respiratory irritation, asthma, allergies, and even cancer.
- High reactivity: Formaldehyde is highly reactive and readily combines with other chemicals.
Applications of Formaldehyde
- Wood industry: Used as an adhesive in the production of fiberboard, plywood, and other wood products.
- Textile industry: Used to make fabrics wrinkle-resistant and as a disinfectant.
- Paper industry: Used to produce odorless and wrinkle-resistant paper.
- Medical industry: Used as a disinfectant and preservative in the production of vaccines and other medical products.
- Production of resins and plastics: Used as a raw material in the production of phenol-formaldehyde resins and other plastics.
Hazards of Formaldehyde
- Human health: Long-term inhalation of formaldehyde can cause eye, nose, and throat irritation, asthma, allergies, and an increased risk of cancer. Direct skin contact can cause burns and sensitivity.
- Environment: The release of formaldehyde into the environment can contribute to air and water pollution and harm plants and animals.
Formaldehyde Production
The Formox process is an industrial chemical process for producing formaldehyde, owned and licensed by Johnson Matthey Chemical Company. Industrially, formaldehyde is produced by the oxidation of methanol in the presence of a silver catalyst or a mixture of iron oxide with molybdenum or vanadium. In the Formox process, iron oxide with vanadium and/or molybdenum is used as a catalyst. In this process, oxygen and methanol react at 300-400 degrees Celsius according to the following equation:
CH₃OH + ½ O₂ → H₂CO + H₂O
The process based on the silver catalyst is usually carried out at temperatures above 650 degrees Celsius. In this method, two chemical reactions occur simultaneously. One reaction is similar to the previous method, and the other involves the dehydrogenation of methanol according to the following reaction equation:
CH₃OH → H₂CO + H₂
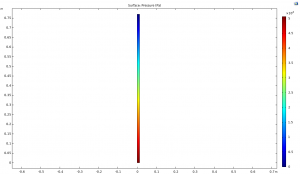
Simulation of Methanol to Formaldehyde Conversion Tower with COMSOL
In this project, the methanol-to-formaldehyde conversion tower is simulated using the COMSOL software. The figure below shows the pressure profile within the tower.