Introduction
Methanol is a colorless, water-soluble liquid with a mild alcohol odor. Since 1800, this feedstock has been extensively used to produce industrial chemicals such as formaldehyde, acetic acid, dimethyl terephthalate, methyl methacrylate, and numerous other chemical compounds. Approximately 60% of methanol consumption is in the chemical industry, while the remaining 40% is used as fuel. Its fuel applications include direct blending with gasoline, as a feedstock for synthesizing methyl tert-butyl ether (MTBE) for gasoline blending, as a feedstock for synthesizing dimethyl ether (DME) for LPG blending, and in the synthesis of gasoline from methanol (MTG).
Methanol Production
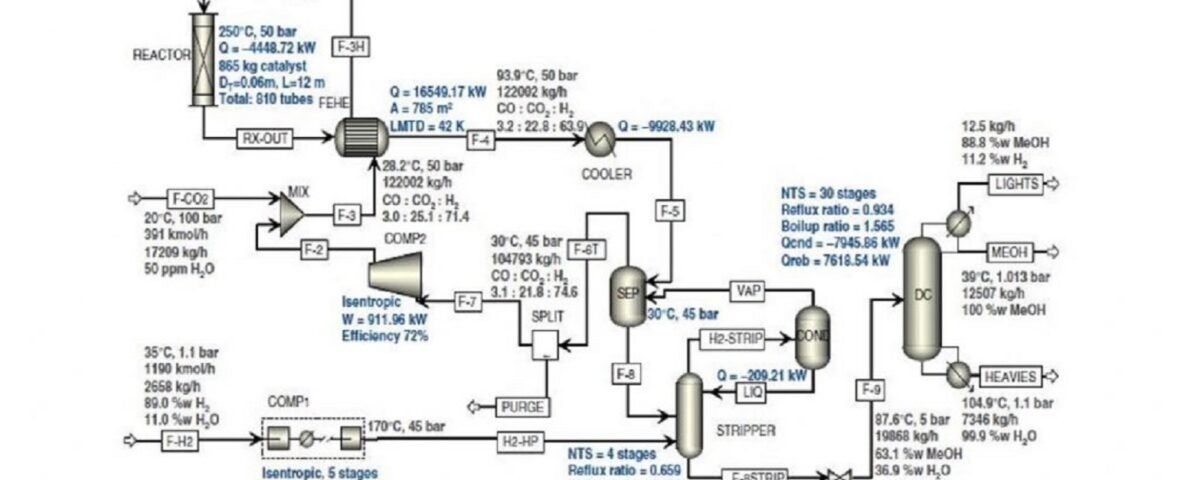
Various methods can be used to produce methanol, but they all generally converge on the following two reactions:
CO + 2 H₂ → CH₃OH CO₂ + 3 H₂ → CH₃OH + H₂O
The primary feedstock for all methanol production units is synthesis gas, a mixture of carbon monoxide (CO), carbon dioxide (CO₂), and hydrogen (H₂). Synthesis gas can be obtained from various sources such as natural gas, coal, biomass, and others. Today, due to its low cost and availability, natural gas is commonly used to produce synthesis gas and subsequently methanol. However, the use of coal is also increasing.
Methanol is one of the three most important products of the chemical industry worldwide, and many substances are derived from it. Given the anticipated shortage of energy resources in the future, the direct use of methanol as a clean fuel or in the production of hydrogen for fuel cells is gaining significant attention.

Primary Applications of Methanol
Methanol, as a strategic commodity, finds extensive use in the production of various end products such as solvents, paints, plastics, and antifreeze. The diversity of methanol derivatives and its application in various industries have established it as a strategic commodity. Consequently, fluctuations in its price significantly impact numerous manufacturing industries.
A substantial portion of the world’s methanol production is dedicated to the synthesis of formaldehyde, acetic acid, and MTBE. Acetic acid (ethanoic acid, CH₃COOH, C₂H₄O₂) or vinegar has a long history. Humans have been producing it through non-synthetic methods, primarily fermentation and distillation, since ancient times. It has been used in the production of food, pharmaceuticals, and industries such as leather tanning and dye manufacturing.
Feasibility Study of a Methanol Plant (Project Design and Economics)
This project was initially simulated using Aspen Plus software, followed by an economic analysis.