Introduction
The identification of optimal processes has become a universal priority among designers worldwide. Given the rapid advancements in science and the emergence of novel processes for producing diverse products from various raw materials with limited capital, this project aims to select and evaluate the most efficient process from among the available options. In this endeavor, the impact of design variables such as reactor volume, distillation column sequence, reflux ratio, and others on product yield and associated costs will be quantified through economic potential calculations.
The subsequent chapters delve into economic considerations and the potential of various design levels to minimize operational costs. Given the potential use of this product as a reactant in numerous processes, environmental concerns will also be addressed to prevent the production of harmful pollutants.

Product Introduction
The primary product under consideration in this project is monoethylene glycol, with by-products including diethylene glycol and triethylene glycol.
Monoethylene Glycol
Monoethylene glycol is an organic compound with the chemical formula (CH2OH)2. It is a colorless, viscous liquid with a sweet taste. It is highly miscible with water and has reasonable miscibility with other light alcohols such as methanol, ethanol, and propanol.
Monoethylene Glycol Production Process
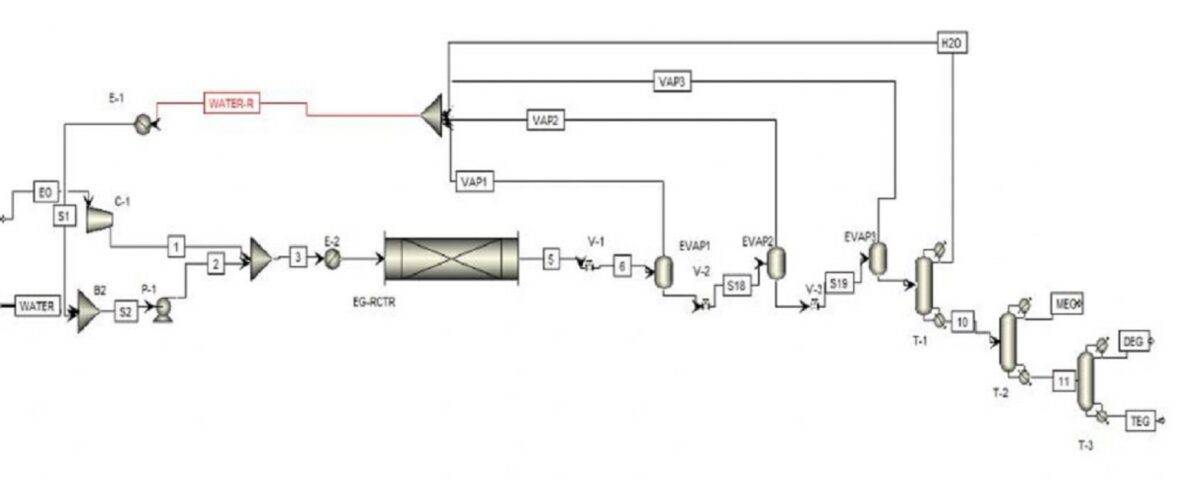
In this process, monoethylene glycol is produced by the reaction of ethylene oxide with a sufficient amount of water in an exothermic reaction. The reaction takes place in the aqueous phase, and while producing monoethylene glycol, by-products such as diethylene glycol and triethylene glycol are produced by the reaction of monoethylene glycol with ethylene oxide. To achieve better selectivity for monoethylene glycol, it is better to feed ethylene oxide and water into the reactor at a mass ratio of 1:7. Also, a temperature of 150°C to 200°C and a pressure of 15 to 20 bar are necessary to initiate the reaction. The hydration reactions of ethylene oxide are as follows:
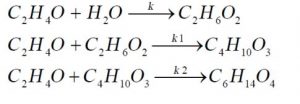
Conceptual Design and Economic Analysis of a Monoethylene Glycol Unit
The main feedstock for the unit is ethylene oxide, which after being compressed by compressor C-1, is mixed with water and the return stream from the evaporators (containing water and monoethylene glycol) and enters exchanger E-1 to be preheated to the reaction temperature before entering the reactor. Since the reaction is exothermic, the reactor outlet is at a higher temperature. Given that the mass ratio of water to ethylene oxide is 6 for optimal selectivity, the reactor effluent contains a significant amount of water. Therefore, a three-stage evaporator is placed after the reactor to separate a portion of the water in the stream and return it to the reactor.
After the evaporators, tower T-1 is used to separate the remaining water in the stream. After water separation, the product stream enters towers T-2 and T-3 to separate monoethylene glycol, diethylene glycol, and triethylene glycol, respectively, and are sent to storage tanks as products. The simulated process flow diagram using ASPEN PLUS software is shown in the following figure.
A conceptual design and economic analysis of a monoethylene glycol unit has been conducted using Aspen Plus software. A comprehensive report is also available.