Introduction
Currently, the most significant method for producing sulfuric acid is the first process, which involves production from elemental sulfur. Sulfur dioxide can be produced from the oxidation of raw sulfur or the oxidation of metal sulfides such as copper sulfide, lead sulfide, nickel sulfide, iron sulfides, zinc sulfide, or molybdenum sulfide. The oxidation of hydrogen sulfide also results in the production of sulfur dioxide. The conversion of sulfur dioxide to sulfur trioxide occurs in a catalytic oxidation process using a cesium or modified vanadium pentoxide catalyst. Finally, sulfuric acid is produced from the hydration of sulfur trioxide.
The primary commercial method for producing sulfuric acid involves initially producing sulfur dioxide from sulfur, The sulfur dioxide is then converted to sulfur trioxide in a catalytic oxidation process, and finally, concentrated sulfuric acid is produced by reacting sulfur trioxide with water.
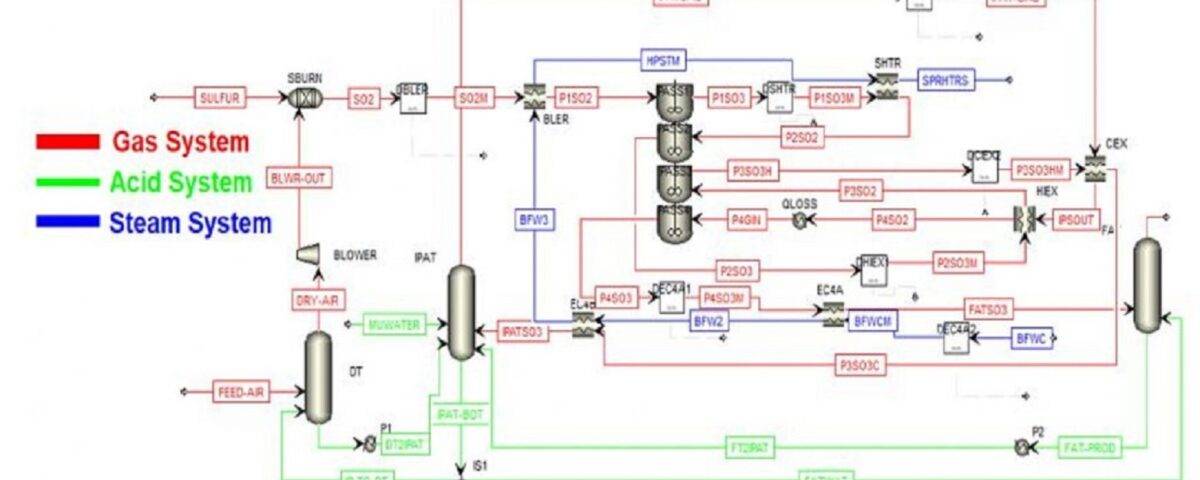
The overall process of producing sulfuric acid using this method is presented in the following diagram:

Sulfur Dioxide as a Byproduct and Subsequent Acid Production
Sulfur dioxide is a common byproduct in the production of copper and other metals. The produced sulfur dioxide is diluted with air and then fed into a catalytic reactor. In this reactor, sulfur dioxide reacts with oxygen to form sulfur trioxide and The sulfur trioxide is then absorbed by sulfuric acid, producing high-concentration (98.5% by weight) sulfuric acid.
Project Objectives
This project aims to perform energy and material balance calculations for two main stages of the process:
- Conversion Reactor R-102: This is the catalytic converter where the majority of the sulfur dioxide is converted to sulfur trioxide.
- Primary Sulfur and Air Mixing Reactor R-101: In this initial reactor, molten sulfur is fed and mixed with dry air, resulting in the production of some SO2 and SO3.
The gas mixture from R-101 is then fed to the catalytic reactor R-102 to achieve an overall conversion of 96%.
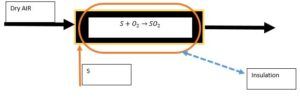
Primary Reactor (Sulfur Burner)
This conversion reactor is adiabatic, and its balances are as follows:
Four-Stage Secondary Conversion Reactor
This reactor operates as a four-stage bed system. However, due to the varying conditions at the inlet and outlet of each bed, each bed must be modeled as an individual reactor, and separate mass balances must be performed for each stage. Each catalytic package has similar conditions, and the governing equations and physics are identical. Therefore, we will first derive the equations for a single catalytic bed and then, for each individual bed, input the corresponding inlet conditions and calculate the subsequent outlet values. It is evident that the outlet of the first stage is the inlet of the second stage, with the difference being that the heat generated is recovered integrally (as indicated in the process flow diagram) along the way. Therefore, the inlet of the second bed is considered after the heat recovery process, not before.
Governing Equations for Each Reactor Section
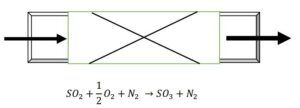
Since the reactor is catalytic and operates adiabatically, the equations for this section are derived with minor modifications similar to the previous reactor case. Assuming we have only one bed:
All balances and calculations are included in the project report file. Additionally, this project includes a training video on how to perform the calculations.