Description
In addition to improving the existing processes for energy production and consumption, the increase in global demand for natural gas also requires pollution control. One of the most serious problems faced by technologies related to natural gas processes. There are large amounts of acid gases that make up about 40% of the gas coming out of the wells. About 46/13 % of natural gas reservoirs in the world contain more than 10% H2S and about 9/26 % of these reservoirs contain more than 10% CO2 gas.
Several methods have been proposed to reduce the amount of acid gases in natural gas. Among them, we can mention chemical adsorption, physical adsorption, hybrid process, surface adsorption using a solid column and using a membrane. Among these methods, the adsorption of acid gases by a liquid solvent (chemical adsorption) is the most common method used in the gas sweetening industry.

Sweetening of Natural Gas
In the 1930s, alkanol amines were used for gas sweetening for the first time. And from then until the 1970s, monoethanolamine (MEA) was the most widely used. But since the 1970s, due to the disadvantages of monoethanolamine, such as corrosion and solvent wastage, diethanolamine (DEA) replaced this amine.
Since the mid-1970s and especially in the last two decades, methyl diethanolamine (MDEA) has been widely used in the gas industry due to its advantages such as: the ability to selectively separate hydrogen sulfide in the presence of carbon dioxide, high stability and low energy consumption for solvent recovery.
Alkanol amines generally consist of at least one hydroxyl group (-COH) and at least one amino group (-NH2). Conventional amines used for gas sweetening. are:
Primary amines, monoethanolamine (MEA) and diglycolamine (DGA)
Secondary amines, diethanolamine (DEA) and diisopropanolamine (DIPA)
Tertiary amines, triethanolamine (TEA) and methyl diethanolamine (MDEA)
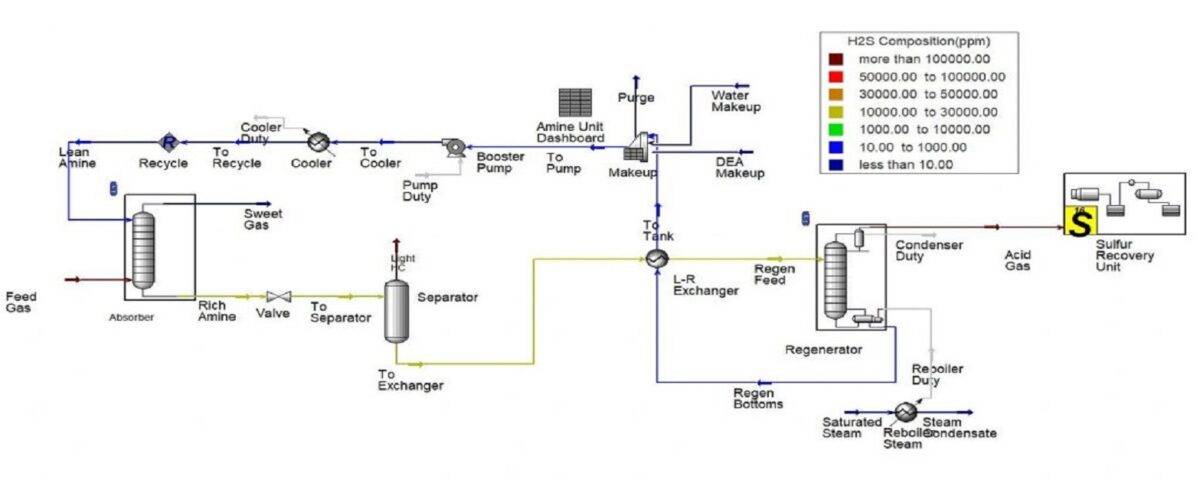
Aspen Hysys Simulation of NG Sweetening Unit With DEA
In this project, the natural gas sweetening unit is simulated with DEA during the 3-step Claus process in Aspen Hysys software.
Diethanolamine is considered a tertiary amine, and as a result, it does not have active amino groups (-NH) in its molecular structure. For this reason, it has a stable chemical structure and selectivity for absorption. In fact, the main factor in the decomposition of type 1 and type 2 amines is the existence of these active amino groups. Decomposed amine is usually very corrosive. This stability of third type amine makes it possible to use its high concentrations (up to 50%) regardless of corrosion problems.
This issue causes that to absorb a certain amount of acid gases, the required flow rate from the third type amine solution is lower than the required flow rate from the first or second type amine solution, and as a result, the amount of thermal energy consumed by the reboilers of the Regeneration Tower is be significantly reduced.