Description
Ethylene is the simplest unsaturated hydrocarbon and is the first member of the alkenes group. Its chemical formula is C2H4, there is a double bond between two carbon atoms. Due to the existence of this double bond, the ethylene isomer has no conformation, that is, the two halves of the molecule cannot change their conformation by rotating around the double bond. In this project, we will simulate the process of producing ethylene from ethanol.
Ethylene Production Methods
Ethylene is produced in the petrochemical industry by steam cracking. In this process, gaseous hydrocarbons and light hydrocarbon solutions obtained from oil are heated for a very short time at a temperature of 750-950 degrees Celsius. Generally, in this reaction, large hydrocarbons are broken into small hydrocarbons, saturated hydrocarbons are converted into unsaturated hydrocarbons by losing hydrogen.
The product of this reaction is a mixture of hydrocarbons, of which ethylene is the main one. The mixture is separated by condensation and fractional distillation. Other methods are hydrogenation of acetylene using a catalyst and dehydration from ethanol.
The reactions of producing ethylene from ethanol are as follows.
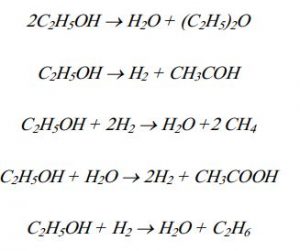
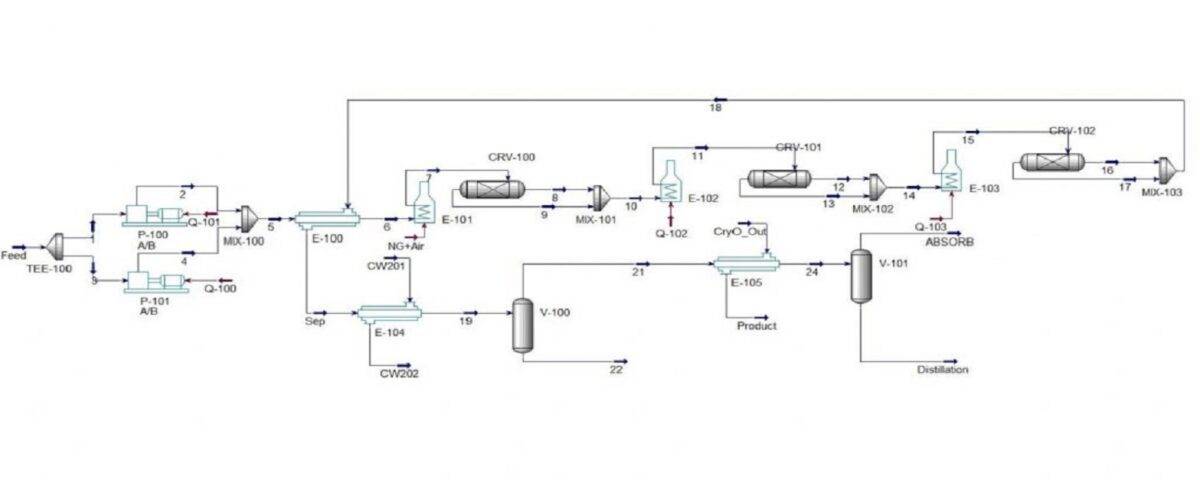
Simulation of Ethylene Production From Ethanol
In this project, the simulation of ethylene production from ethanol has been done in Aspen Hysys software.