Introduction
The oil refining industry faces numerous challenges in today’s world, including compliance with environmental regulations and improving fuel quality. One of the primary concerns is the reduction of sulfur compounds in fuel oil to minimize environmental pollutants and enhance refinery efficiency. In this regard, advanced technologies such as Steam Methane Reforming (SMR), Hydrogen Separation (PSA), Hydrodesulfurization (HDS), and Sulfur Recovery (SRU) serve as key solutions for desulfurization in fuel oil processing. This project aims to design a multi-stage process chain and perform an accurate simulation to propose an integrated system for improving fuel oil quality and Fuel Oil Sulfur Removal pollutants.
Project Objectives
The project primarily focuses on Fuel oil desulfurization, with the following key objectives:
- Enhancing Fuel Oil Quality: Fuel Oil Sulfur Removal to provide fuel that meets higher environmental standards.
- Increasing Energy Efficiency: Optimizing operations through heat recovery and energy reutilization across different process units.
- Optimizing Operational Conditions: Fine-tuning parameters such as temperature, pressure, and stream composition based on international standards like API and reference sources.
- Developing Advanced Technologies: Implementing cutting-edge processes such as SMR, PSA, HDS, and SRU to achieve optimal sulfur removal and refinery performance.
Technologies and Process Chain
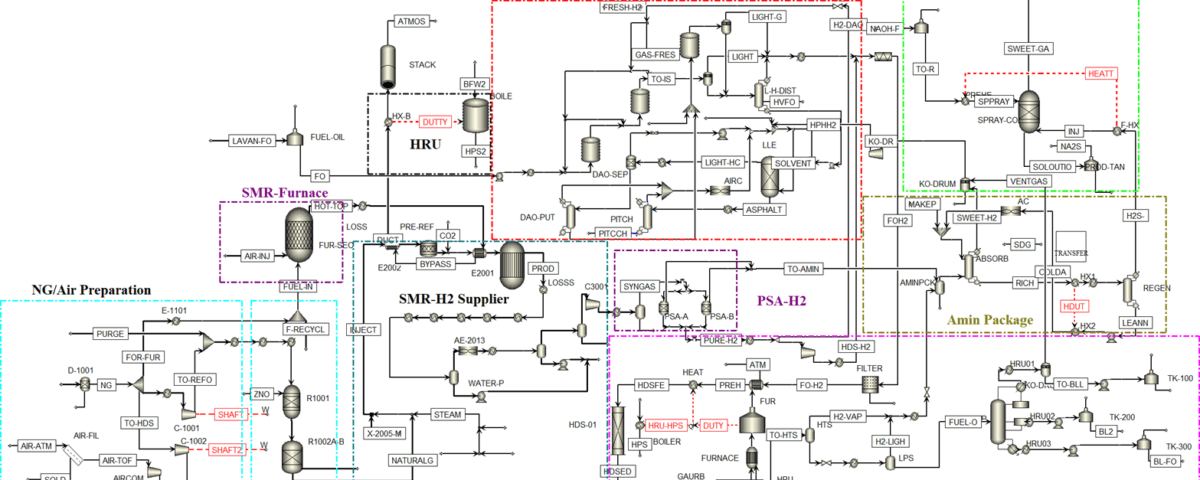
A multi-stage integrated system has been designed based on conventional industrial practices, comprising the following sections:
-
NG/AIR Preparation:
The initial stage involves purifying the feedstock (natural gas and air) from microscopic contaminants, particulate matter, and excess moisture. Proper conditioning of the feed ensures optimal performance in subsequent processing stages.
-
NG Desulfurization – ZnO Bed:
Activated ZnO beds are used to react with sulfur compounds in natural gas. The reaction converts H₂S into ZnS, preventing sulfur from entering critical units such as the SMR furnace.
-
SMR-Furnace (Steam Methane Reforming):
In the SMR furnace, methane reacts with steam at high temperatures to produce hydrogen and carbon monoxide. This stage serves as the primary hydrogen source for subsequent hydrogenation reactions.
-
Heat Recovery Unit (HRU):
Waste heat from SMR exhaust streams is recovered and repurposed to preheat feedstock for various process units. This strategy enhances energy efficiency and reduces fuel consumption.
-
SMR-H₂ Supplier:
The hydrogen produced in the SMR furnace is separated from other gases and purified for use in hydrodesulfurization.
-
Fuel Oil Deasphalting Unit – LCMAX Process:
Heavy fuel oil is treated using the LCMAX process to remove impurities and asphaltic compounds, improving its physical properties and preparing it for hydrogenation.
-
PSA-H₂ Separation:
Hydrogen from SMR undergoes Pressure Swing Adsorption (PSA) to separate it from other gases, ensuring a high-purity hydrogen supply for downstream processing.
-
Fuel Oil HDS-A/B with Guard Bed and Dilution Stream:
Treated fuel oil, along with high-purity hydrogen, enters the hydrodesulfurization reactor. A guard bed and dilution stream control the reaction and prevent impurity buildup, effectively reducing sulfur levels to meet regulatory limits.
-
SRU-Na₂S, NaHS Unit (Sulfur Recovery):
Sulfur extracted from fuel oil is recovered in the form of byproducts such as sodium sulfide (Na₂S) and sodium hydrogen sulfide (NaHS), which can be reused in chemical industries.
-
Amin Package:
Acidic gases are removed using amine-based absorption technology. Amines selectively capture H₂S and CO₂, purifying the final gas stream.
This project exemplifies an innovative process chain design for fuel oil desulfurization. By integrating advanced technologies, the proposed system enhances fuel quality, improves energy efficiency, and significantly reduces environmental pollutants. This approach serves as a model for optimizing refinery operations and aligning with international environmental standards.
Technical Description of the Process
1. NG/Air Preparation
Purpose and Importance of the Process
In this unit, the feedstock, consisting of natural gas and air, is prepared for subsequent processing stages. The removal of particulates, moisture adjustment, and initial purification play a crucial role in enhancing the performance of downstream units, including the steam methane reformer (SMR) and hydrogen separation systems. Optimizing the feed conditions not only prevents damage to sensitive equipment but also increases overall process efficiency.
Operational Steps and Equipment Used
Particulate Removal: Advanced filtration systems are utilized to eliminate dust and suspended particles from the natural gas and air feed. These filters are designed in compliance with international standards.
Moisture Adjustment: Drying or moisture separation systems are employed to remove excess water and achieve the appropriate moisture level, which is critical due to the sensitivity of catalysts in the SMR process.
Pressure Regulation: Precise pressure control is maintained using compressors and pressure regulators to ensure that operational conditions meet the requirements of downstream processes.
Proper execution of the feed preparation operations results in a cleaner and optimally conditioned feedstock, significantly improving the performance and longevity of subsequent processing units. Moreover, adherence to API standards and chemical engineering guidelines ensures accurate operational conditions aligned with industrial best practices.
2. Desulfurization of Natural Gas Using ZnO Bed
Importance of Removing Sulfur Compounds from Natural Gas
Sulfur compounds, particularly hydrogen sulfide (H₂S), in natural gas can cause various issues, including equipment corrosion, catalyst poisoning, and reduced efficiency in refining processes. Therefore, one of the critical stages in feed preparation for downstream processes is sulfur removal using specialized adsorbents such as zinc oxide (ZnO). This technology is a proven method for purifying gases containing hydrogen sulfide and other sulfur contaminants.
ZnO Bed Mechanism
At this stage, the purified natural gas passes through a ZnO-containing bed, where the removal of H₂S occurs under controlled temperature conditions. The primary reaction in this process is as follows:
ZnO + H₂S → ZnS + H₂O
In this reaction, ZnO reacts with H₂S, converting it into ZnS, which remains as an immobile solid. This process ensures that the remaining sulfur in the feedstock is minimized, preventing its entry into the steam methane reforming (SMR) unit.
Advantages of ZnO-Based Sulfur Removal
- Extended SMR Catalyst Life: Preventing sulfur poisoning effects.
- Enhanced Gas Feed Quality: Reducing interfering compounds for downstream processes.
- Reduced Equipment Corrosion: Preventing the formation of corrosive sulfur compounds.
- Lower Operating Costs: Minimizing maintenance and premature equipment replacement.
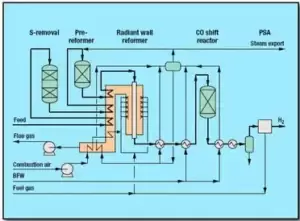
3. Steam Methane Reforming (SMR)
Steam methane reforming (SMR) is one of the most critical and widely used industrial processes for hydrogen production. In this method, methane from natural gas reacts with steam to produce a significant amount of pure hydrogen. Due to its high efficiency, relatively low cost, and broad industrial application, SMR is considered the primary method for hydrogen supply in refineries and chemical industries.
Fundamental SMR Reactions
The SMR unit involves two key reactions occurring over metallic catalysts such as nickel (Ni) at high temperatures:
Steam Methane Reforming Reaction:
CH₄ + H₂O → CO + 3H₂
This endothermic reaction requires high temperatures and moderate pressure. The reforming furnace provides the necessary energy to break down methane and generate hydrogen.
Water-Gas Shift (WGS) Reaction:
CO + H₂O → CO₂ + H₂
In this stage, carbon monoxide (CO) reacts with steam to produce additional hydrogen along with carbon dioxide (CO₂). This reaction occurs at moderate to low temperatures and plays a crucial role in increasing hydrogen yield.
Key Operational Stages in the SMR Unit
- Preheating and Feedstock Mixing: Natural gas (mainly methane) and steam are mixed in the correct ratio and preheated in heat exchangers to achieve the optimal initial temperature for reactions.
- Reaction in the Reforming Furnace: The gas-steam mixture enters the reforming furnace, where the SMR reaction takes place in the presence of nickel catalysts. High temperatures and moderate pressure conditions are maintained.
- Cooling and Entry into the WGS Reactor: The reformer effluent, containing H₂, CO, CO₂, and steam, is cooled before entering the WGS reactor to enhance hydrogen production.
- Hydrogen Separation and Purification: The produced hydrogen is transferred to the PSA separation unit, where impurities such as CO, CO₂, and N₂ are removed, yielding hydrogen with a purity of over 99.99%.
SMR Process Advantages and Challenges
Advantages:
- High efficiency in industrial hydrogen production
- Lower costs compared to alternative methods such as water electrolysis
- Compatibility with refineries and petrochemical industries for hydrogen feedstock supply
Challenges:
- CO₂ emissions as a byproduct (requiring optimization to minimize environmental impact)
- High energy consumption and the need for efficient catalysts to improve process yield
Applications of SMR-Derived Hydrogen
- Utilization in hydrodesulfurization (HDS) and refinery hydrogenation units
- Feedstock for ammonia and methanol production
- Use in fuel cells and clean fuel production
SMR remains the dominant industrial method for hydrogen production, playing a vital role in meeting the needs of refineries and petrochemical plants. Optimizing this process, including heat recovery and reducing carbon emissions, can significantly enhance refinery efficiency and operational cost savings.
4. Heat Recovery Unit (HRU)
Importance of Heat Recovery in Refining Processes
Energy recovery from hot process streams is a fundamental principle in refinery and petrochemical plant design. In SMR, a significant amount of heat is released from the reformer exhaust gases. If not recovered, this heat is lost as wasted energy. The heat recovery unit (HRU) employs advanced technology to effectively capture and repurpose this waste heat, improving energy efficiency, reducing operational costs, and minimizing fuel consumption.
HRU Working Mechanism
The HRU is designed based on heat transfer principles and utilizes heat exchangers. The hot exhaust gases from the SMR furnace pass through a series of heat exchangers, transferring energy to other process fluids. Key applications of this recovered energy include:
- Preheating the SMR feedstock: Increasing the temperature of methane and steam before entering the reactor.
- Heating process fluids in downstream units: Providing necessary energy for hydrogen separation in the PSA unit.
- Generating steam for turbines or other refinery units: Enhancing overall energy efficiency.
Benefits of HRU Implementation
- Reduced fuel consumption: Less reliance on additional energy for feedstock preheating.
- Improved energy efficiency: Enhanced overall refinery performance.
- Lower greenhouse gas emissions: Optimized energy usage leading to reduced CO₂ emissions.
- Extended equipment lifespan: Minimizing thermal stress and temperature fluctuations in process systems.
5. Hydrogen Separation Unit via PSA
Role of Hydrogen in Refineries and Petrochemical Industries
Hydrogen is a crucial component in refining and petrochemical industries, playing a key role in hydrotreating, catalytic reforming, and ammonia and methanol production. The SMR process is one of the primary industrial methods for hydrogen generation. However, the output gas contains H₂, CO₂, CO, and minor traces of steam and methane. To obtain pure hydrogen, pressure swing adsorption (PSA) technology is utilized.
PSA Process for Hydrogen Separation
PSA is based on selective adsorption using materials such as zeolites and activated carbon, which can capture unwanted gases while allowing hydrogen to pass through. The process involves:
- Feed gas enters adsorption beds.
- Selective adsorption of CO₂ and CO.
- Desorption and regeneration of adsorption beds.
- Collection of high-purity hydrogen (>99.99%).
Benefits of PSA for Hydrogen Production
- High hydrogen purity for critical processes
- Cost-effective and efficient separation technology
- Flexible operation with adjustable pressure settings
- No need for chemical solvents, reducing environmental impact
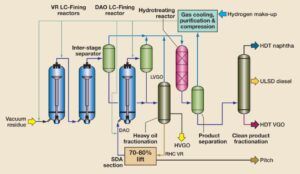
6. Heavy Oil Deasphalting Unit – LCMAX Process
Importance of Deasphalting Heavy Fuel Oil
Heavy fuel oil contains significant amounts of heavy organic compounds such as asphaltenes, resins, metallic compounds, nitrogen, and sulfur compounds. These components increase viscosity, reduce thermal value, and cause operational challenges in refinery units. The LCMAX process is an advanced method for removing these compounds and enhancing the physical and chemical properties of heavy fuel oil.
Operating Principles of the LCMAX Process
The LCMAX process is based on solvent extraction, utilizing light solvents such as propane, butane, or selected petroleum-based solvents to separate asphaltenes and other heavy compounds. The key steps of this process include:
- Heavy Fuel Oil Feed Intake: The heavy fuel oil is collected from distillation columns or other refining processes and introduced into the deasphalting unit.
- Solvent Mixing: Light solvents are injected into the fuel oil, dissolving the lighter components while asphaltenes and other heavy compounds remain as a separate phase.
- Phase Separation: By adjusting operational conditions (temperature and pressure), two distinct phases are formed: deasphalted oil (DAO) and asphalt precipitate.
- Solvent Recovery: The utilized solvent is recovered from both phases and recycled back into the process, reducing operational costs.
- DAO Preparation for Downstream Processing: The deasphalted oil (DAO) is prepared for further processing in the hydrodesulfurization (HDS) unit, where sulfur and nitrogen compounds are removed using hydrogen.
Advantages of the LCMAX Process in Heavy Oil Refining
- Reduction of viscosity and improvement of feedstock quality for downstream units
- Enhanced efficiency of hydrogenation processes with lower hydrogen consumption
- Mitigation of operational challenges such as deposit formation in heat exchangers and reactors
- Economic value enhancement by removing undesirable compounds
- Potential use of DAO in base oil and lighter fuel production
The heavy oil deasphalting unit (LCMAX) plays a crucial role in refining heavy fuel oil by reducing asphaltenes and heavy metals. This process increases the efficiency of downstream processes such as hydrogenation (HDS) and prevents operational problems like deposit formation in equipment.
7. Hydrodesulfurization of Fuel Oil (Fuel Oil HDS-A/B with Guard Bed and Dilution Stream)
Importance of Hydrodesulfurization in Fuel Oil Refining
Fuel oil, as one of the heavy products of refineries, contains high amounts of sulfur, nitrogen, oxygen compounds, and heavy metals. These impurities lead to the formation of pollutant gases such as SO₂ during combustion, causing significant environmental concerns. Hydrodesulfurization (HDS) is one of the most efficient methods for sulfur removal, enabling the production of low-sulfur fuels.
Hydrodesulfurization Process Steps
In this unit, pre-treated fuel oil is introduced into hydrodesulfurization reactors, where it reacts with pure hydrogen in the presence of catalysts, converting sulfur compounds into H₂S. The key steps of this process include:
- Feed Pre-Treatment and Conditioning: Treated fuel oil, along with a dilution stream and pure hydrogen, enters the reactor. The dilution stream helps control reaction temperature and prevents undesirable deposit formation.
- Guard Bed: Before entering the main catalyst bed, a guard bed containing active adsorbents removes metallic impurities and other catalyst-deactivating contaminants. This step extends catalyst life and improves process performance.
- Hydrogenation Reaction: In this stage, sulfur, nitrogen, and oxygen compounds react with hydrogen under moderate temperature and high pressure, converting into lighter compounds and H₂S gas.
- Separation of Reaction Gases: After leaving the reactor, reaction products are sent to downstream units for separation. H₂S gas is extracted and sent to the sulfur recovery unit (SRU).
Benefits of Hydrodesulfurization of Fuel Oil
- Reduction of sulfur content in heavy fuels and minimization of environmental pollution
- Enhancement of economic value and marketability of refined products
- Improvement of fuel oil stability and quality for use in power plants and industries
- Prevention of deposit formation in equipment, increasing the lifespan of heat exchangers and boilers
The hydrodesulfurization unit (HDS-A/B) plays a vital role in reducing sulfur in heavy fuels. With its guard bed and dilution stream, it effectively eliminates impurities, ensuring high efficiency.
8. Sulfur Recovery Unit (SRU – Na₂S, NaHS Unit)
Necessity of Sulfur Recovery in Refineries
The hydrodesulfurization process generates large amounts of hydrogen sulfide (H₂S) gas, which must be effectively removed and recovered. In the Sulfur Recovery Unit (SRU), H₂S is converted through controlled reactions into valuable products such as sodium sulfide (Na₂S) and sodium hydrogen sulfide (NaHS).
Sulfur Recovery Process and Byproduct Production
- Conversion of H₂S to Sodium Sulfides: H₂S gas is introduced into a specialized reactor and reacts with alkaline solutions such as sodium hydroxide (NaOH), forming sodium sulfide (Na₂S).
- Oxidation Process and Purity Control: In certain cases, part of Na₂S is further oxidized in the presence of air or oxygen to produce sodium hydrogen sulfide (NaHS), which has extensive applications in the paper, leather, chemical, and water treatment industries.
Benefits of Sulfur Recovery as Na₂S and NaHS
- Prevention of sulfur emissions into the environment
- Reuse of sulfur compounds in downstream industries
- Increased refinery efficiency and reduced pollutant disposal costs
- Reduced environmental impact and improved pollution control systems
The sulfur recovery unit (SRU-Na₂S, NaHS) effectively converts H₂S into useful compounds like sodium sulfide and sodium hydrogen sulfide, reducing sulfur emissions while enhancing the refinery’s economic value.
9. Amine Package
Importance of Acid Gas Removal in Refineries
Refinery processes produce gas streams containing significant amounts of acidic compounds such as H₂S and CO₂. These gases must be removed before atmospheric release to comply with environmental regulations, prevent equipment corrosion, and enhance downstream processing efficiency.
Operating Principles of the Amine Package
- Absorption of Acid Gases with Amine Solvents: In this process, gas streams containing H₂S and CO₂ pass through amine solutions such as monoethanolamine (MEA), diethanolamine (DEA), or methyl diethanolamine (MDEA). These solvents selectively absorb acidic gases, producing a high-quality treated gas stream.
- Amine Regeneration and Separation: The amine solution containing absorbed gases is sent to the regeneration unit, where it is heated to release the acid gases, allowing the amine solvent to be recovered and reused.
Advantages of the Amine Package in Refineries
- Reduction of acidic gas emissions, ensuring environmental compliance
- Improvement of gas stream quality and enhanced refinery efficiency
- Lower operational costs through solvent recovery and reuse
- Protection of equipment from corrosion caused by acidic gases
The Amine Package is an efficient technology for final acid gas removal, selectively absorbing H₂S and CO₂, improving gas quality, and reducing the refinery’s environmental footprint.
Tools and Key Project Outcomes
Process Simulation and Modeling Tools
In this project, advanced chemical engineering software was utilized for the simulation, analysis, and optimization of various fuel oil refining processes. These tools enabled a precise assessment of operational unit performance and the impact of different parameters on efficiency, energy consumption, and pollutant reduction. Process simulations and thermodynamic modeling of various compounds in desulfurization, hydrogenation, and sulfur recovery units were conducted using Aspen Plus. This software played a crucial role in process design, simulation, and optimization, providing accurate data for technical and economic decision-making.
Key Project Outcomes
Optimization of Desulfurization Processes
One of the most significant achievements of this project was the optimization of desulfurization processes in hydrodesulfurization (HDS) and sulfur recovery (SRU) units. The analysis results indicate:
- Increased desulfurization efficiency in HDS reactors has led to a reduction in sulfur content in the final products.
- The use of a guard bed in hydrogenation reactors has minimized catalyst fouling and extended its lifespan.
- Optimization of the amine absorption process has improved H₂S and CO₂ separation efficiency while reducing operational costs.
- Enhanced performance of steam methane reforming (SMR) furnaces and increased hydrogen production rates have reduced dependency on external hydrogen sources.
Reduction in Operational Costs and Energy Consumption
- Energy consumption in process units has been reduced through the implementation of a heat recovery unit (HRU), which recycles heat from exhaust gases for feed preheating.
- Hydrogen consumption costs have been minimized by employing Pressure Swing Adsorption (PSA) technology for high-purity hydrogen separation from gas streams.
- Maintenance and equipment repair costs have been reduced through optimized design and the use of corrosion-resistant materials.
Environmental Compatibility Enhancement and Emission Reduction
- SO₂ emissions have been significantly reduced compared to conventional refining processes by improving the efficiency of the sulfur recovery unit (SRU).
- The quality of refined fuel oil has been upgraded to meet global standards (IMO 2020), with lower sulfur content and reduced emissions from combustion.
- Greenhouse gas emissions have been lowered by optimizing combustion processes and improving energy efficiency.
Significance of the Project as an Advanced Technical Solution
This project serves as a scientific and technical reference for fuel oil refineries and can be a foundation for the development of advanced technologies in this field. The most notable achievements include:
- Providing accurate and practical simulation models for both existing and new refineries.
- Developing optimized solutions to reduce operational costs and improve refinery process efficiency.
- Assisting refineries in meeting international environmental standards.
- Enhancing equipment reliability and lifespan by mitigating the effects of corrosion and sulfur deposition.
These solutions can serve as a model for future projects and be adopted as an industrial benchmark for fuel oil refineries worldwide.
Related Project
Design, Simulation and Technical Proposal for Fuel Oil Desulfurization at Lavan Refinery
Conclusion
The refining and optimization of fuel oil using advanced technologies have not only resulted in environmental benefits and improved product quality but have also led to enhanced efficiency, reduced operational costs, and higher refining standards. The optimization of hydrogenation (HDS), desulfurization (SRU), hydrogen separation (PSA), and heat recovery (HRU) processes has played a pivotal role in boosting refinery performance, extending equipment lifespan, lowering energy consumption, and ensuring greater compliance with environmental regulations.
SANILCO, leveraging state-of-the-art refining technologies and an engineering-driven approach, has introduced innovative solutions for improving refinery efficiency and producing low-sulfur fuels. This project serves as an implementable industrial model, paving the way for fuel oil refineries globally to achieve optimal performance, cost savings, and adherence to international standards.
By implementing these solutions, refineries can enhance their productivity, lower operational expenses, and remain committed to international environmental obligations. SANILCO continues to pioneer the development of innovative refining technologies, playing a vital role in shaping the future of the energy industry.