Introduction
Nitric acid is a strong acid with high oxidizing properties. When heated at atmospheric pressure, it boils at 78.2°C and begins to decompose, eventually forming a 68% acidic solution. This mixture forms an azeotrope with a maximum boiling point of 120°C. Pure 100% nitric acid is unstable and decomposes rapidly; in general, the more dilute the acid, the more stable it is. This project simulates the nitric acid production process and conducts an economic analysis.
Pure nitric acid is a colorless liquid, but it turns brownish when dissolved gases (nitrogen oxides) are present. The primary applications of nitric acid include the production of nitrogen-based fertilizers and military applications. Nitric acid is also used as an intermediate in the polymer industry, particularly in the production of polyamides and polyurethanes. It is used for purifying heavy metals (uranium, lead, silver, etc.) and in the plastics, ester (by reaction with alcohols), and salt (by reaction with bases) industries.
Simulation of Nitric Acid Production Process and Economic Analysis
Given the strategic importance of nitric acid, it is essential to evaluate various production processes and compare them to select the optimal process based on Iranian conditions. In this project, the nitric acid production process is simulated using Aspen Plus and Aspen Hysys software. The economic analysis of this process is performed manually.
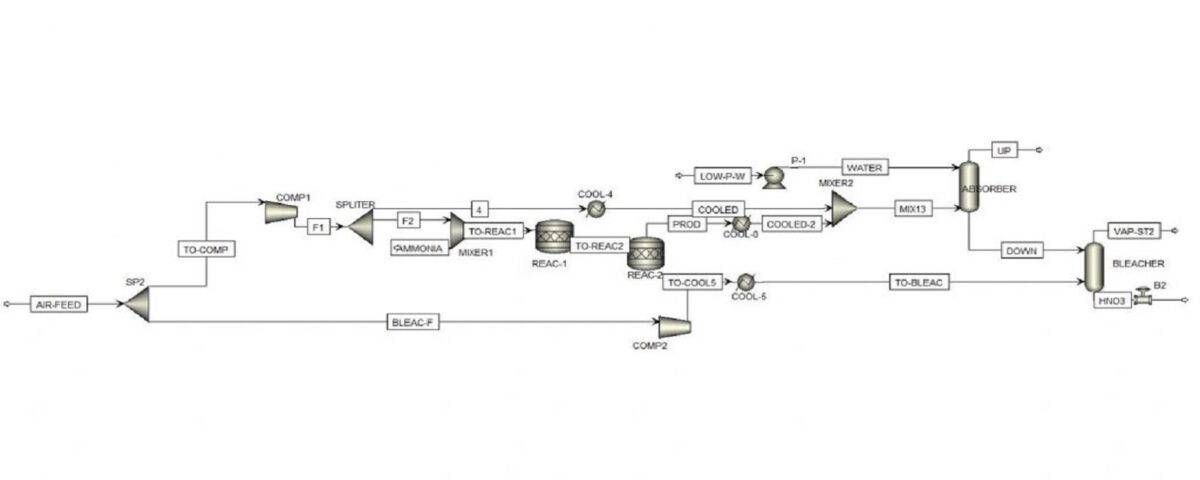
Simulation with Aspen Hysys software
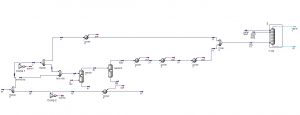
Simulation with Aspen Plus software