Introduction
2-Ethylhexanol is a flammable liquid that can ignite when in contact with strong oxidizing agents. Appearing as a colorless, clear liquid, it is classified as an alcohol and is largely insoluble in water but highly soluble in most organic solvents. This branched, eight-carbon chiral alcohol is produced on a massive scale for various applications, including solvents, flavors, fragrances, and as a precursor in the production of other chemicals such as plasticizers. It is immiscible with water and has a lower density than water. This project focuses on the conceptual design and simulation of the 2-ethylhexanol production process.
2-Ethylhexanol Production Process
The production of 2-ethylhexanol (EH-2) can be carried out in either the gas or liquid phase. If the reactants, hydrogen and 2-ethylhexenal, are introduced into the reactor as a gas phase, the reactor becomes two-phase. If hydrogen is introduced in the gas phase and 2-ethyl hexenal in the liquid phase, the reactor becomes three-phase. One advantage of the gas-phase system is the ability to regenerate the catalyst. Most EH-2 production units operate in the liquid phase, and more studies have been conducted on liquid-phase processes compared to gas-phase processes.
Feasibility Study and Simulation of 2-Ethylhexanol Production Process
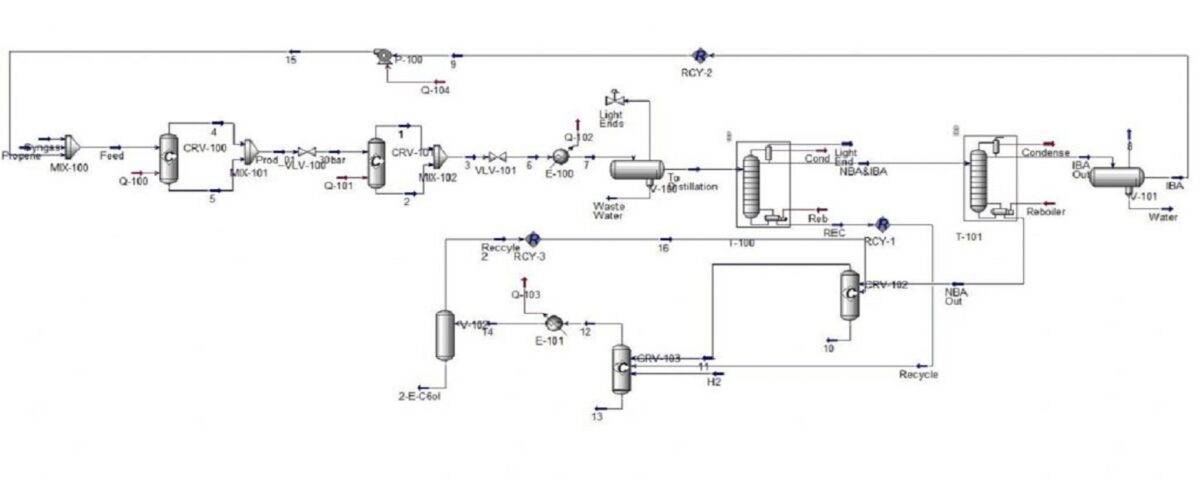
In this project, the process of producing 2-ethylhexanol from propylene and synthesis gas has been carried out with Aspen Hysys software and The reactions for the production of 2-ethylhexanol are as follows.
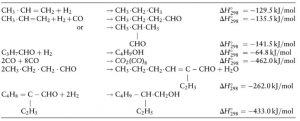
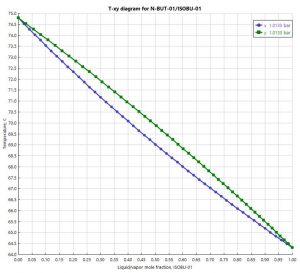
The reaction is carried out in a two-phase reactor. The figure below shows the T-xy diagram made with Aspen Plus.