Introduction
Unlike traditional ethylene production processes that rely on steam cracking of naphtha, direct methane conversion technologies offer a promising alternative. However, these processes, which require high temperatures and specific catalysts, have yet to fully mature and attract significant investor interest. Silurian’s OCM technology, for instance, shows potential to compete with steam cracking, especially when ethane prices are relatively high. A pilot-scale demonstration of this technology has been conducted at the Braskem complex in the United States.
While the Siluria OCM process is still under development, its potential for direct conversion of raw materials into petrochemical products is significant. The abundant supply of natural gas in the United States, driven by shale gas production, provides a favorable environment for the growth of this technology. Moreover, the global availability of natural gas resources and the increasing trade in liquefied natural gas (LNG) can further accelerate the commercialization of this technology.
Methods for Producing Ethylene from Methane
Given methane’s high stability, breaking its molecular bonds requires substantial energy input. This is achieved through a process known as methane pyrolysis, which involves subjecting methane to extremely high temperatures to induce dehydrogenation and subsequent coupling, forming two-carbon products.
Published reports on methane pyrolysis indicate that high ethylene and acetylene yields are only attainable at temperatures exceeding 1200 degrees Celsius and short gas residence times. Consequently, large-scale implementation of this method is economically challenging.![]()
Alternative methods can be categorized into two approaches: direct and indirect conversion.
Indirect Conversion of Methane
In this approach, methane is initially converted into synthesis gas, a mixture of hydrogen and carbon monoxide. This syngas can then be further processed into various valuable products. Synthesis gas is produced through steam reforming:![]()
Direct conversion of methane
Research in the field of direct conversion of methane (without synthesis gas production) expanded in the 80s. The following processes are the most important direct methods. These processes are potentially more energy efficient than indirect methods. The reason for this is the lack of synthesis gas production stage, which requires a lot of energy and high temperature.
Partial oxidation of methane
Methane in the vicinity of oxygen is transformed according to the following reaction: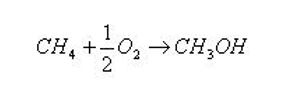
Methanol Production and Its Challenges
In the aforementioned reaction, the produced methanol exhibits lower stability compared to methane when exposed to oxygen. Consequently, the overall products tend to convert into carbon oxides. To maintain methanol selectivity at around 80%, methane conversion must be kept low (approximately 8%), making this process economically unattractive.
Methane Coupling with Chlorine
Methane and chlorine can be combined in various ways to produce valuable hydrocarbons. One method is the Pistengburg process, where chlorine, methane, and oxygen react in a fluidized bed reactor at 16 atmospheres and 350°C over a copper catalyst.
Methane chloride, when reacted over a zeolite catalyst, yields a mixture of hydrocarbons, including high-octane gasoline. The low temperature and high methane conversion (around 50%) make this process feasible. However, the economic challenge lies in the significant amount of HCl produced and released from the reactor.
Non-equilibrium Plasma
A recent research focus in converting methane into heavier hydrocarbons involves the use of non-equilibrium plasma. The reactor consists of two metal electrodes made of aluminum plates, one of which is covered by a dielectric plate. The gas passes through the dielectric and the other aluminum plate. By applying a high voltage at ambient temperature, methane can be converted into heavier hydrocarbons. One advantage of this method is the absence of the need for oxygen in the reaction.
Oxidative Coupling of Methane (OCM)
Since 1982, Union Carbide has been at the forefront of research aimed at directly converting methane into valuable products such as ethylene, propylene, and liquid transportation fuels. By 1993, over 950 publications and patents on this topic had been reported. Research in OCM covers various areas, including homogeneous reactions, catalysts, mechanisms, economics, and future prospects.
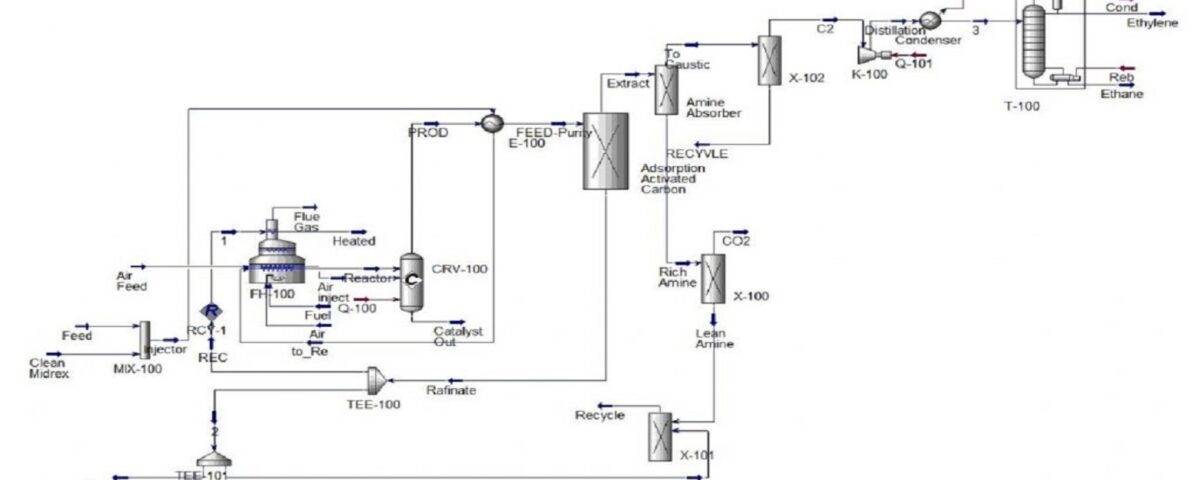
Feasibility Study for Ethylene Synthesis Unit from Methane Oxidation with Purification Unit
In this project, the ethylene synthesis unit from methane oxidation has been simulated with Aspen Hysys software and its economic analysis has been done.The figure below shows a brief description of the process.
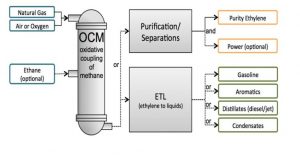
This project has a complete report.