Introduction
Fossil fuel resources worldwide are rapidly depleting while global energy consumption continues to rise. This has increased the need for alternative and new energy sources. Fossil fuels currently account for 80% of the world’s primary energy supply and cause significant environmental damage.
Shifting from conventional energy sources to renewable energy is an urgent need to prevent catastrophic consequences. However, new energy sources must be capable of replacing fossil fuels both quantitatively and qualitatively, Hydrogen with its high energy capacity, is expected to be a suitable solution to this problem.
Hydrogen, a Secondary Energy Carrier: Produced from primary energy sources like fossil fuels (coal, oil, natural gas), nuclear power (fission and breeder reactors), and renewable energy (hydropower, solar, wind, and biomass), hydrogen serves as a versatile energy carrier.
Both versions are correct and effective and The choice between them depends on the specific context and the target audience.
Hydrogen Application
Hydrogen has numerous applications across various industries.In the refining industry, hydrogen is particularly important for purifying and converting other products and Refinery processes that consume hydrogen include:
- Hydrodesulfurization
- Hydrogenation processes
- Hydrocracking
- Hydrocracking of heavy compounds
- Fuel applications
- Hydrogen and fuel cells
The use of renewable energy is not a choice but a necessity. Achieving sustainable development is impossible without considering advanced technologies and modern tools. The increasing global demand for energy, coupled with the depletion of fossil fuel resources, has placed the world in a significant challenge to secure its future energy supply.
In this context, a new perspective on the use of hydrogen has emerged due to its unique properties. Hydrogen’s advantages include abundance, very low pollutant emissions, the reversibility of its production cycle, and reduced greenhouse gas effects. Using hydrogen as a fuel reduces environmental pollutants and eliminates sulfur oxides and carbon oxides resulting from fossil fuel combustion.
One way to convert hydrogen into the most common types of energy needed, such as electricity and heat, is through the use of fuel cells. Fuel cells are energy converters that directly convert the chemical energy of a fuel into electrical energy. The primary fuel for these cells is hydrogen or other hydrogen-related gases such as natural gas or methanol. When these gases are burned, only water and heat are produced, reducing environmental pollutants and eliminating sulfur and carbon oxides resulting from fossil fuel combustion. This technology is unparalleled in terms of not producing pollutants such as nitrogen oxides, carbon monoxide, and unburned hydrocarbons.
Fuel Cells
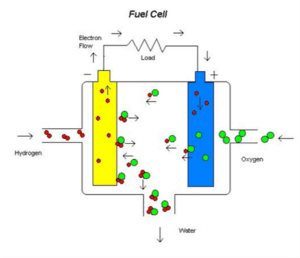
All fuel cells have a similar appearance. Two electrodes, the anode and cathode, form its components, wrapped around an electrolyte (solid or liquid). An external circuit connects the two electrodes and converts the electrons around the circuit into an electric current. In fact, the chemical reaction between oxygen and hydrogen elements in the fuel cell produces clean energy. The only byproduct of this process is water.
In the provided diagram, an oxidation reaction occurs at the anode of the hydrogen fuel cell. The electrons produced enter the external circuit and then move to the cathode. The positive ions produced at the anode pass through the electrolyte to the cathode, where they combine with oxygen from the air and the electrons that have entered the cathode from the external circuit, forming water in the presence of a catalyst.
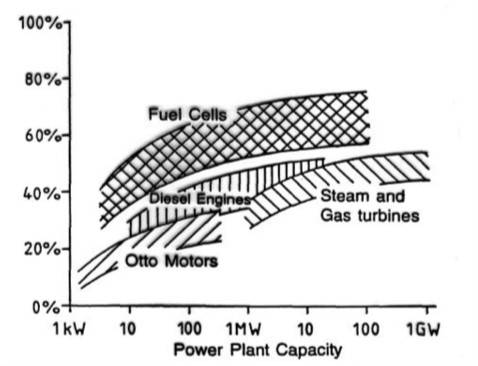
Fuel cells do not obey the laws governing heat engines; therefore, their efficiency can reach up to three times that of heat engines. Depending on the type and design, the electrical efficiency of fuel cells is about 40 to 60% (based on the lower heating value). The efficiency of a fuel cell is constant and independent of its size. When the output heat is also utilized, the efficiency can reach approximately 85%.
Due to their high efficiency and the absence of solid particulate emissions, fuel cells can reduce carbon dioxide emissions by up to 50% compared to conventional power generation methods. This makes them an attractive technology for the clean utilization of fossil fuels. Other significant advantages include a very long lifespan, negligible noise pollution, reduced fuel consumption, and more, promising a bright future for this technology.
Hydrogen Production Methods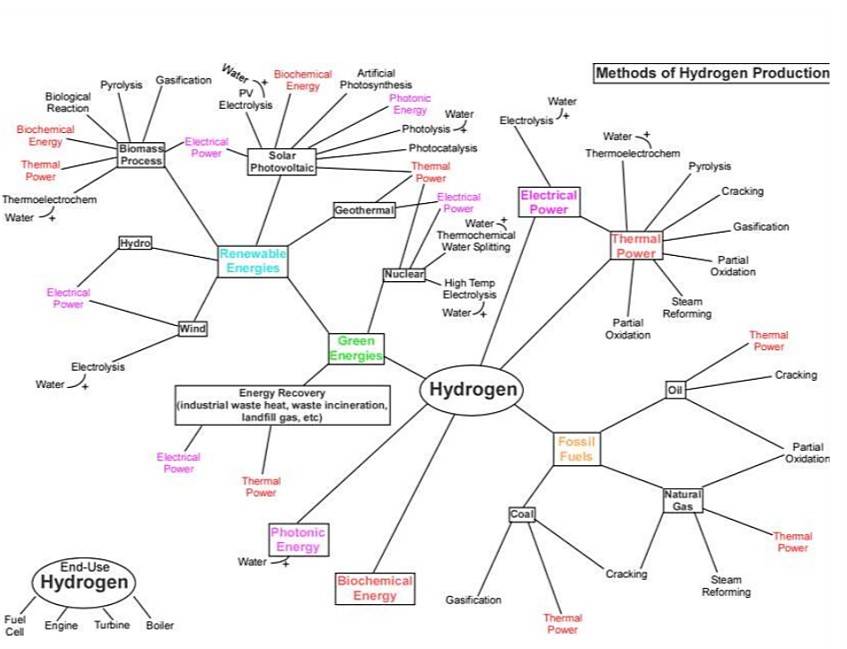
Hydrogen Production from Natural Gas
Steam Methane Reforming
Steam methane reforming is a process where natural gas or any methane-containing stream such as biogas or landfill gas, propane, butane, pentane, and light and heavy naphtha reacts with steam in the presence of a catalyst to produce hydrogen and carbon dioxide.
Autothermal Reforming of Hydrocarbons
Partial oxidation is an alternative to steam methane reforming, often preferred for higher hydrocarbons or when pure oxygen is available.
Hydrogen Production from Coal
Coal, a fossil fuel, can be converted into hydrogen gas through a process known as coal gasification. This process involves reacting coal with steam and oxygen at high temperatures to produce a mixture of gases, including hydrogen, carbon monoxide, and carbon dioxide.
Hydrogen Production by Electrolysis
Electrolysis offers a clean method for producing hydrogen without carbon or sulfur impurities Several electrolysis methods exist, including alkaline water electrolysis, proton exchange membrane electrolysis, solid oxide electrolysis cells, and high-temperature water electrolysis, among the most common and advanced While there are various methods for producing hydrogen from renewable energy sources, electrolysis is currently the most practical approach.
Ion Exchange Membrane Method
Although water electrolysis has been known for about 200 years and can easily produce ultra-pure hydrogen, the high power consumption of electrolyzers makes it less competitive with other large-scale technologies in terms of production cost. As a result, only 5% of total hydrogen production comes from this method.
Hydrogen Production from Biomass via Thermochemical Conversion Processes
Biomass could be the most suitable organic substitute for oil in the future،Biomass can be derived from various sources such as animal waste, municipal solid waste, residues of agricultural products, fast-growing trees, agricultural waste, sawdust, aquatic plants, fast-growing grass species like grass, waste paper, corn, and many more. Biomass can be converted through various technical processes such as anaerobic digestion, fermentation, metabolic processing, supercritical water treatment, species formation, artificial photosynthesis, hydrolysis.
Process Description
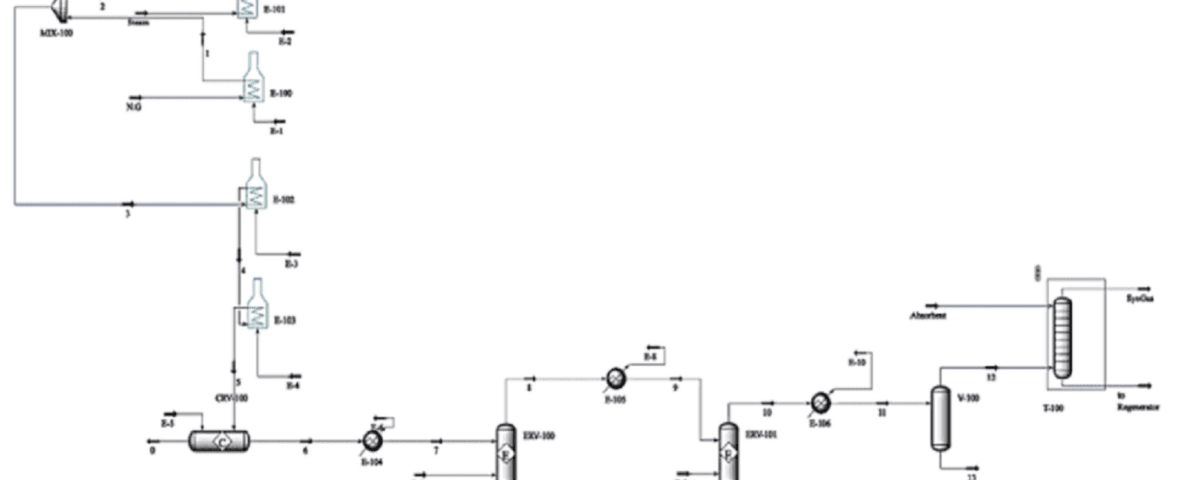
Hydrogen production from natural gas is one of the most common methods for producing this versatile element. This process, known as steam methane reforming (SMR), involves several chemical reactions.
Main Reactions:
- Steam methane reforming: In this primary reaction, methane (CH4), the main component of natural gas, reacts with steam (H2O) at high temperatures (700 to 1100 degrees Celsius) in the presence of a catalyst (usually nickel) to produce hydrogen (H2) and carbon monoxide (CO).
- Water-gas shift reaction: This reaction is used to convert carbon monoxide (CO) to hydrogen (H2) and carbon dioxide (CO2).
Side Reactions:
- Methanation: In this reaction, carbon monoxide (CO) reacts with hydrogen (H2) to produce methane (CH4) and water (H2O).
- Boudouard reaction: In this reaction, methane (CH4) and steam (H2O) react to form carbon dioxide (CO2) and hydrogen (H2).
Advantages of hydrogen production from natural gas:
- Abundance: Natural gas is an abundant and relatively inexpensive fossil fuel.
- Reliability: The technology for producing hydrogen from natural gas is well-developed and mature, and is very reliable in operation.
- Centralized production: Hydrogen can be produced centrally in large units, which can make its transportation easier.
- Diverse applications: Hydrogen is used in a wide range of applications, including power generation, transportation, refining, and chemical production.
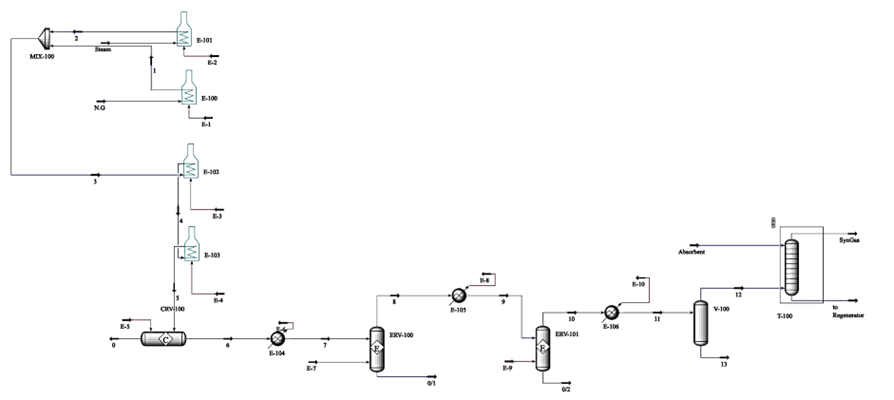
Simulation of hydrogen production from natural gas Simulation of the process to assess the economic feasibility of the process to the end of the clean fuel production process was performed in Aspen HYSYS version 12.1.
Technical and Economic Calculations of Hydrogen Production Process
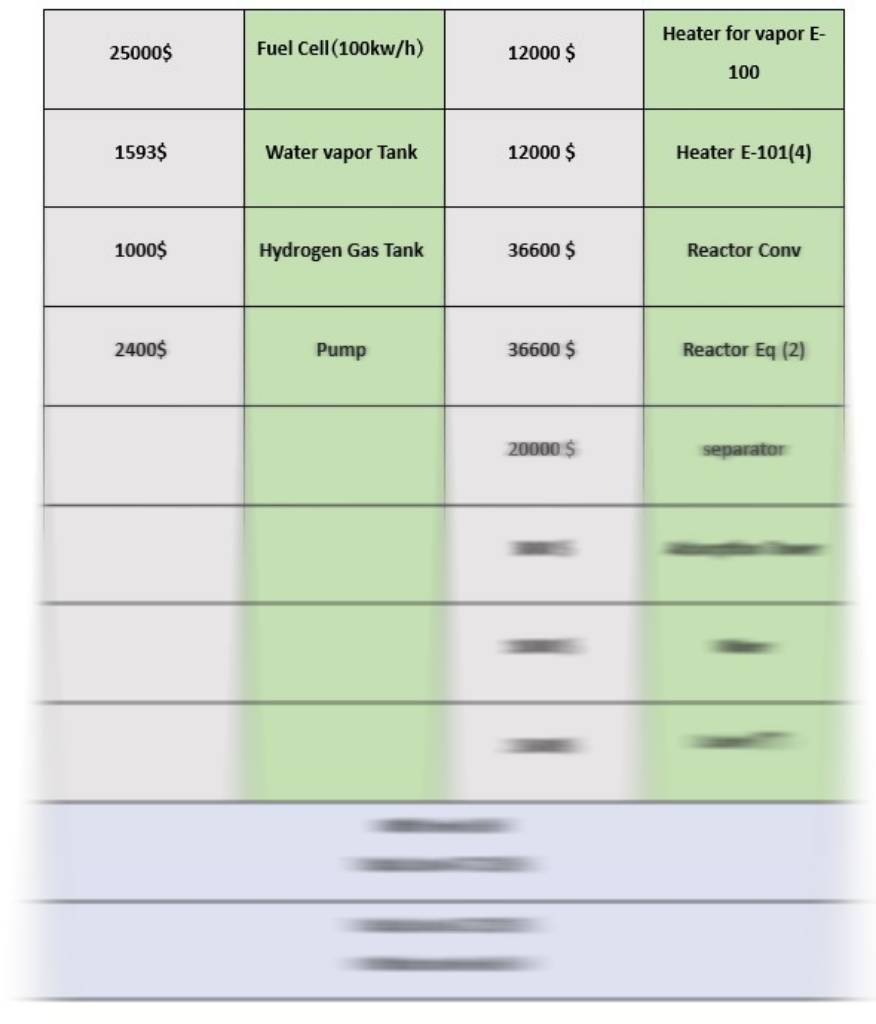
The following equipment is considered for economic calculations:
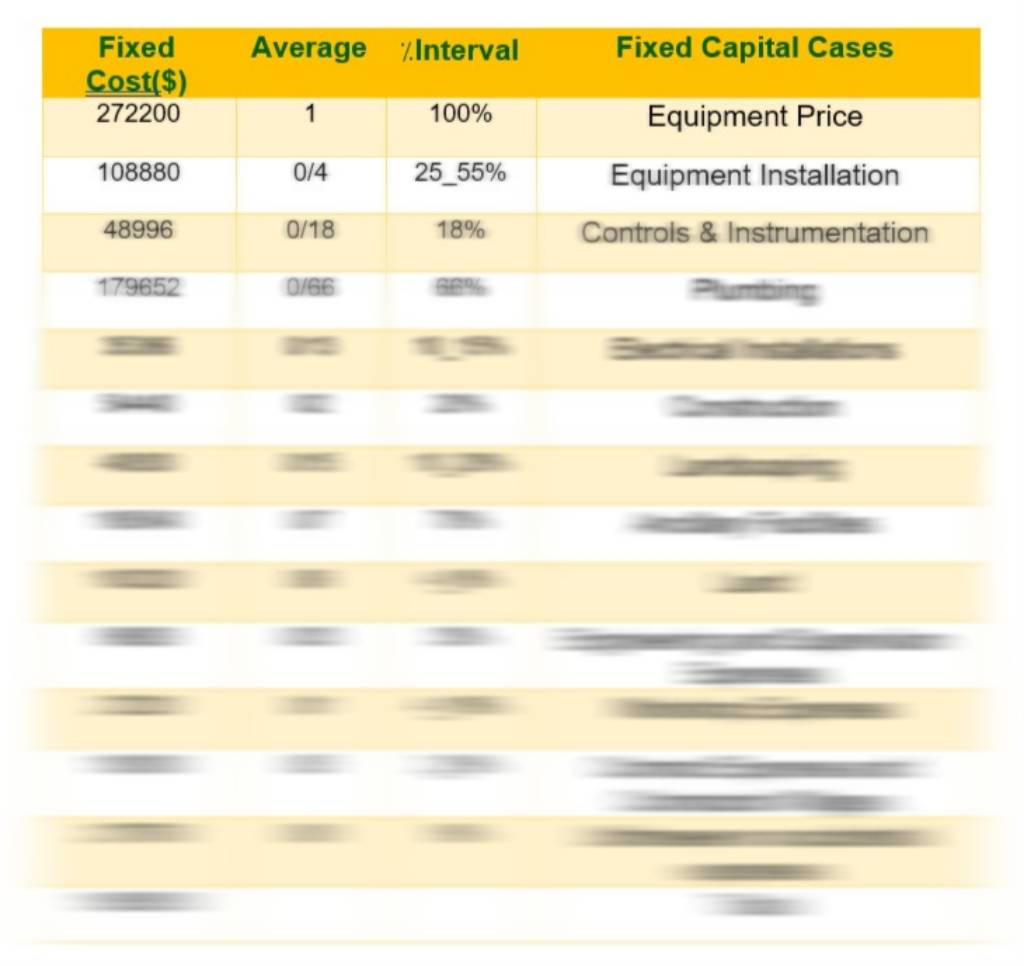
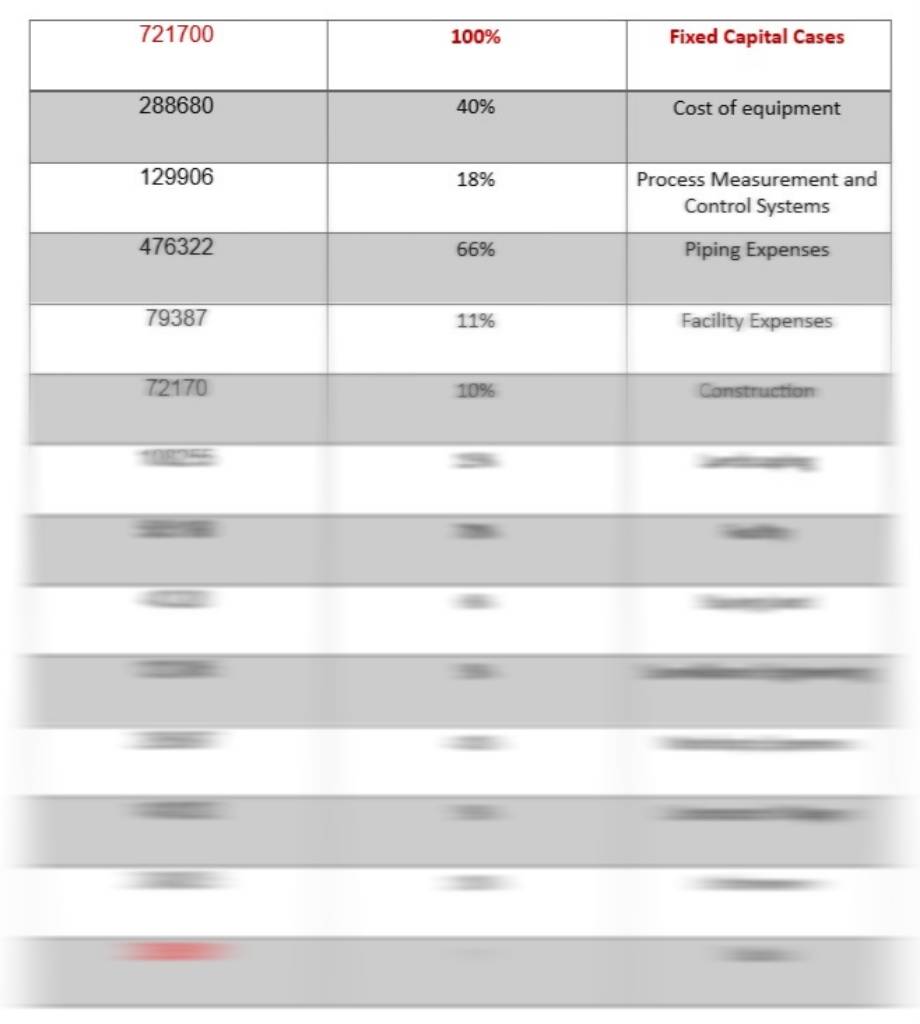
And also calculations of TPC, FCI including fuel cell and without fuel cell
Conclusion
As you know, hydrogen has attracted significant attention from researchers and industries as a clean and efficient energy carrier. One of the common methods for producing hydrogen is steam reforming of natural gas. Fuel cells are devices that directly convert the chemical energy of hydrogen into electrical energy.
Simulation and economic analysis of this process allow us to find the optimal operating conditions for producing high-purity hydrogen at low cost and also to evaluate the overall efficiency of the hydrogen-powered power generation system.
Simulation and Economic Analysis of Hydrogen Production from Natural Gas for Fuel Cells
In this project, the simulation and economic analysis of the hydrogen production process from natural gas for use in fuel cells were performed using Aspen HYSYS version 12.1 software.