Introduction
Dimethyl ether (DME), the simplest aliphatic ether, is a colorless, ether-like odor, non-toxic, highly flammable gas that is soluble in water and alcohol. Also known as methoxymethane, DME is an isomer of ethanol. Physically, DME exhibits properties similar to liquefied petroleum gases (LPG) such as propane and butane, existing as a gas under normal temperature and pressure but liquefying under moderate pressure and cooling. Due to its high oxygen content and the absence of sulfur and other harmful compounds, DME is classified as a clean fuel. DME can be converted into olefins and synthetic hydrocarbons.
Application of DME
DME serves as a valuable precursor for synthesizing organic compounds. For instance, dimethyl sulfate can be produced using dimethyl ether and sulfur trioxide. Dimethyl ether can be transformed into acetic acid through the Monsanto process, an industrial method involving the catalytic carbonylation of methanol.
As a laboratory reagent and solvent, DME is a useful extraction agent due to its low boiling point, allowing for easy separation from reaction mixtures. A mixture of Dimethyl ether and propane is used for wart treatment. DME finds applications in hairspray, adhesives, microwaves, and as a refrigerant alongside Ammonia, Carbon dioxide, Butane, and Propene.
Owing to its low soot emission and non-toxic nature, DME is employed as a substitute for propane in liquid fuels, methanol, diesel, and gasoline. Additionally, DME is utilized in power plant fuels, fuel cells, automotive fuel, electricity generation, heating, and cooking.
Industrial and Laboratory Methods for Dimethyl Ether Production
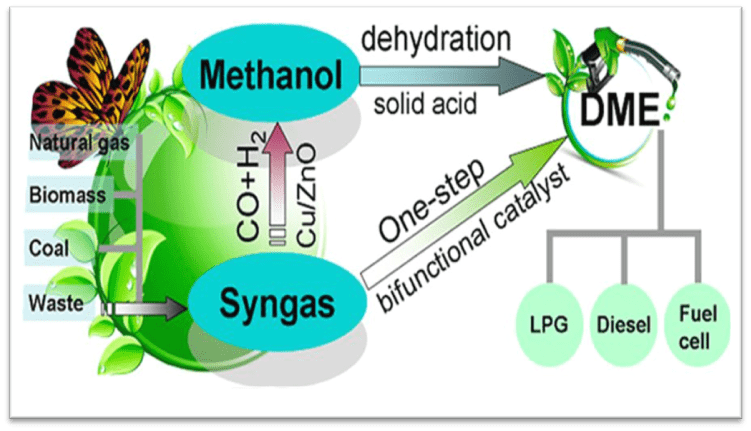
Catalytic synthesis of dimethyl ether (DME) in the gas phase can be broadly classified into two main categories: direct and indirect methods.
Direct Method
In this method, synthesis gas (a mixture of carbon monoxide and hydrogen) is converted into dimethyl ether in the presence of dual-function catalysts. Common catalysts for this reaction include those based on copper or cobalt.
CO + 2H₂ → CH₃OH
This method is currently under development and is not fully optimized for industrial use.
Advantages of the Indirect Method:
- Simplicity: Requires only one reactor and one catalyst.
- Higher efficiency: Can achieve higher DME yields compared to the indirect method.
- Lower cost: Can be generally cheaper to operate compared to the indirect method.
Disadvantages of the Direct Method:
- Sensitivity to reaction conditions: Direct DME reactions are highly sensitive to temperature, pressure, and H₂/CO ratio, requiring precise control of these parameters to achieve optimal results.
- Formation of byproducts: Undesired byproducts such as methane, ethane, formaldehyde, and acetic acid may form, necessitating the separation and purification of the final DME.
Indirect Method
In the indirect method, synthesis gas is first converted to methanol in the presence of catalysts such as zinc oxide, copper oxide, and alumina. Subsequently, dimethyl ether is obtained from the dehydration of methanol in the presence of solid acid catalysts.
2CH₃OH → CH₃OCH₃ + H₂O
This method is used in industrial settings and is fully developed.
Advantages of the Indirect Method:
- Greater catalyst diversity: A wider range of catalysts can be used for both reaction steps.
- Possibility of methanol separation: Methanol can be extracted as a separate intermediate product and used for other purposes.
Disadvantages of the Indirect Method:
- Greater complexity: This method involves two separate reaction steps and requires two different types of catalysts.
- Lower overall yield: Overall DME yield is generally lower than in the direct method.
- Higher cost: Due to its greater complexity, this method can be more expensive than the direct method.
From a Chemical Engineering Perspective:
The choice between the direct and indirect methods for DME production depends on various factors, including feedstock availability, desired product purity, economic considerations, and environmental regulations. While the direct method offers potential advantages in terms of simplicity and efficiency, the indirect method is currently more mature and widely used in industry. Ongoing research and development efforts are focused on improving the performance of direct synthesis catalysts and addressing the challenges associated with this method.
Simulation of DME Production Process
The simulation of the process in order to evaluate the economy of the process until the end of the biofuel production process has been carried out with Aspen Hysys software version 12.1.
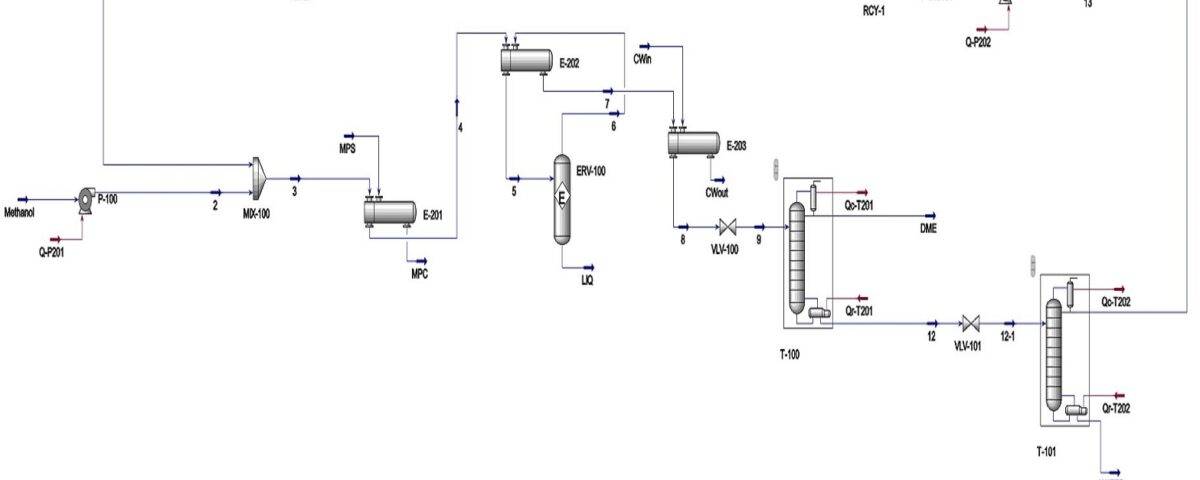
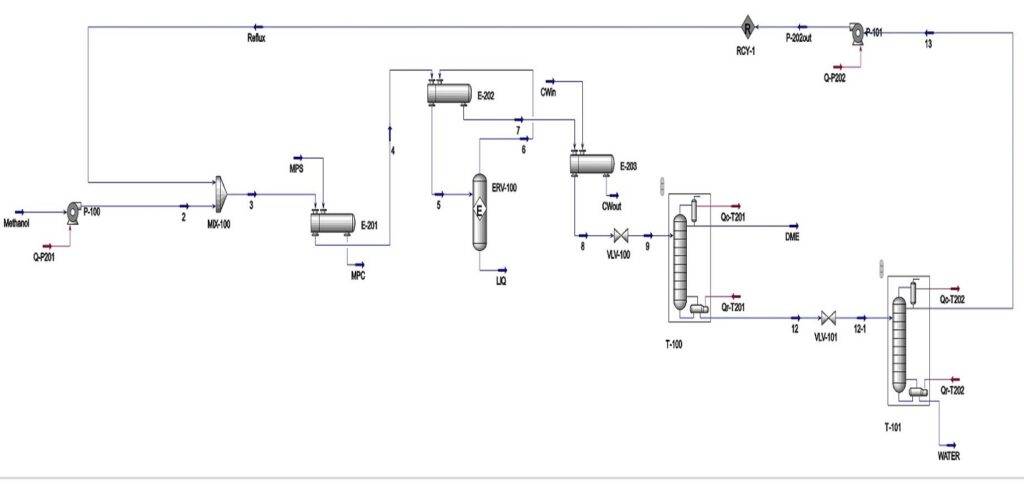
Process Description
Feed Introduction and Preheating
The feedstock, methanol, is introduced into the process at a flow rate of 1.767 tons per hour. It is then pumped to a pressure of 15 bar and mixed with a methanol recycle stream in a mixer. To reach the reaction temperature, the mixture passes through two shell-and-tube heat exchangers, increasing its temperature to 250°C.
Note: To leverage the exothermic nature of the reaction, the hot outlet stream from the reactor is used to preheat the incoming feed in the second heat exchanger, instead of steam.
Reaction and Cooling
The preheated feed enters the reactor. Due to the exothermic nature of the reaction, the temperature rises to 376°C at the reactor outlet.
Cooling and Pressure Reduction
To prepare the reactor effluent for the distillation column, it is cooled in a third heat exchanger. As the stream has already been partially cooled in the second heat exchanger, less cooling duty is required in the third one.The cooled stream is then pressure reduced to 10.4 bar using a control valve and fed into the tenth tray of a 22-tray distillation column. DME is separated from methanol and water and sent to storage tanks.
Methanol Recovery
The bottom stream from the first column, containing water and methanol, is further pressure reduced to 7.4 bar and fed into the fourteenth tray of a second distillation column with 26 trays. Methanol is recovered from the top of this column, pumped to 15 bar, and recycled back to the beginning of the process.
Technical and Economic Calculations of the Process Unit
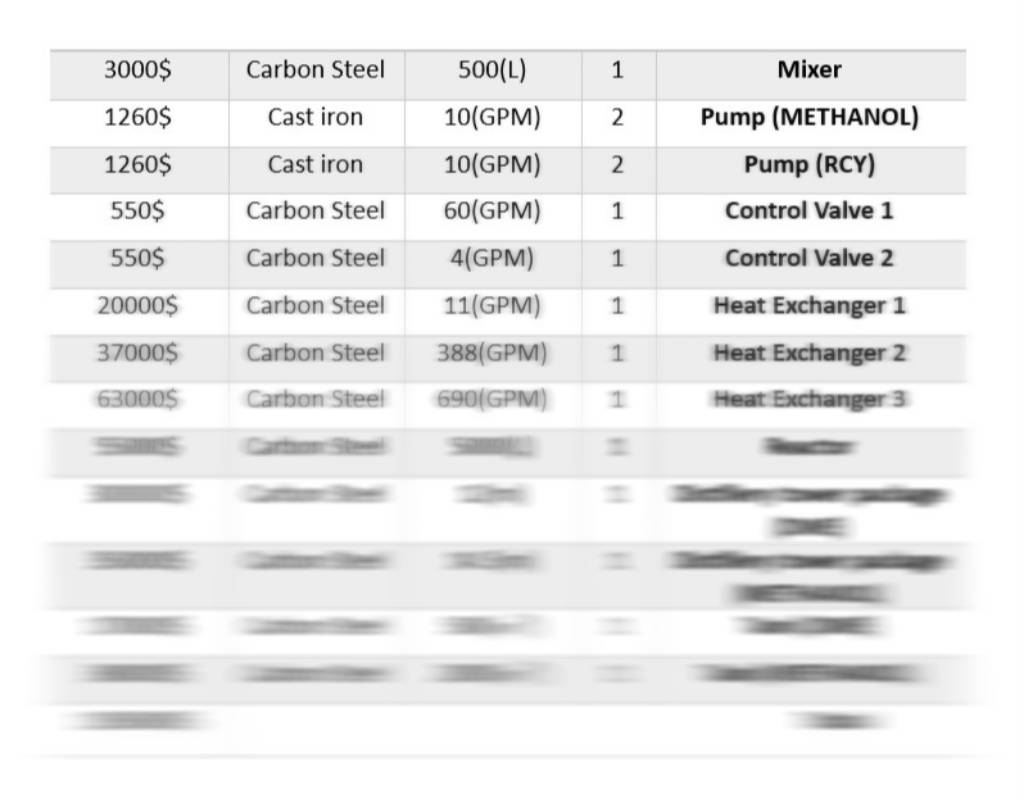
The following equipment is considered in economic calculations:
Equipment Information Table
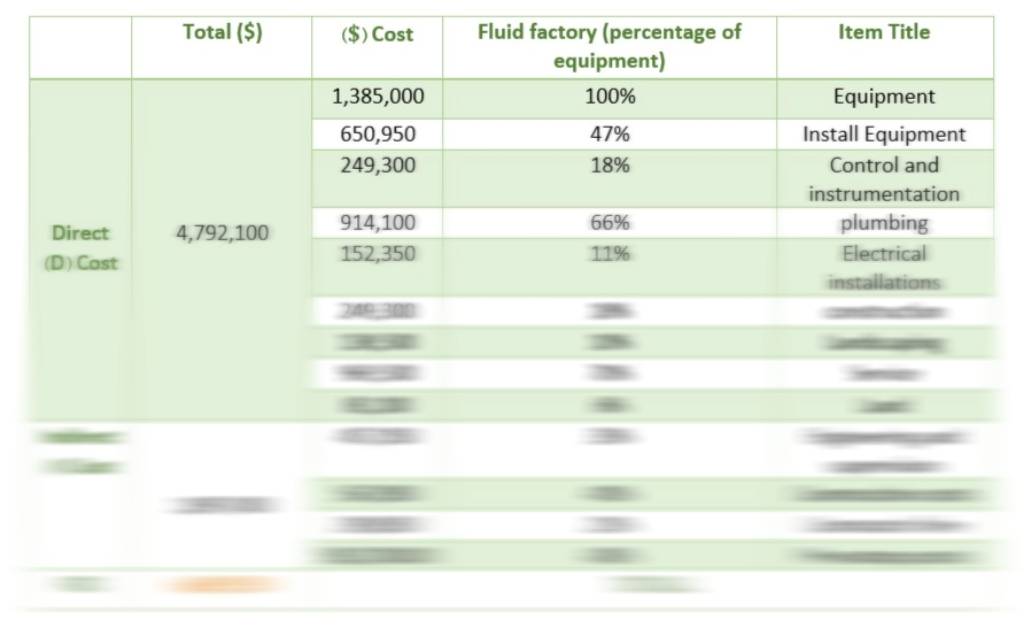
Economic Calculations
Having the FCI, you will subsequently be able to obtain the TPC and WCI.
Conclusion
In today’s world, with the increasing demand for clean and efficient fuels, dimethyl ether (DME) has gained attention as a clean and efficient alternative to fossil fuels. The DME production process involves complex stages such as synthesis gas synthesis, methanol production, and methanol dehydration. To better understand this complex process and optimize it, powerful simulation software such as Aspen HYSYS is used.
Economic Analysis and Simulation of the Dimethyl Ether (DME) Production Process with Aspen HYSYS
In this project, the simulation and economic analysis of the DME process have been carried out using Aspen HYSYS version 12.1.