Introduction
Synthesis gas, hydrogen and carbon dioxide absorption are three key concepts in the field of energy and chemical industries, which are strongly related to each other. These concepts play a fundamental role in the development of clean technologies and reduction of harmful environmental effects. Synthesis gas, as a valuable chemical intermediate, consists of a combination of carbon monoxide and hydrogen. This gas is of great importance due to its ability to be converted into a wide range of chemical products and clean fuels.
Hydrogen is known as the cleanest fuel and has the potential to replace fossil fuels in various energy sectors. This gas has wide applications in fuel cells and chemical industries. Carbon dioxide adsorption has also been proposed as a solution to deal with climate change. This process includes removing carbon dioxide from the atmosphere and storing it in underground reservoirs or turning it into value-added products.
Description of The Process
Syngas is a mixture consisting mainly of hydrogen (H2), carbon monoxide (CO) and often carbon dioxide (CO2). It is an essential intermediate in the production of various chemicals and fuels such as ammonia, methanol and synthetic hydrocarbons through processes such as Fischer-Tropsch synthesis.
1. Synthesis Gas Production Methods:
– Steam methane reforming (SMR): the most common method, in which natural gas (mainly methane) reacts with steam at high temperature (700 to 1000 °C) in the presence of a catalyst to produce synthesis gas. The reaction is endothermic and can be represented as follows:
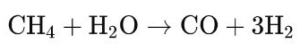
– Partial oxidation: In this process, a hydrocarbon raw material is partially oxidized with a limited amount of oxygen and produces synthesis gas in an exothermic reaction.
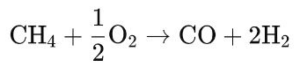
– Coal gasification: solid coal is converted into syngas by reacting with oxygen and steam at high temperature. This method is especially important in areas with abundant coal resources.
– Biomass Gasification: similar to coal gasification, but using biomass as raw material. This process is considered as a more sustainable alternative.
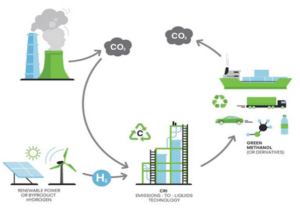
2. Synthesis Gas Composition and Uses:
– The ratio of hydrogen to carbon monoxide in syngas varies depending on the production method and is often adjusted for specific downstream applications. For example, a high H2/CO ratio is favorable for ammonia production. While a lower ratio may be preferred for liquid fuel.
Adsorption of Hydrogen and Carbon Dioxide:
In many industrial processes, including the production and use of synthesis gas, it is necessary to separate and purify hydrogen and carbon dioxide from the gas mixture. This separation is usually achieved through absorption processes.
1. Hydrogen Adsorption:
– Pressure Swing Adsorption (PSA): This is the most common method for hydrogen purification. Synthesis gas passes through a bed of absorbent materials (such as zeolites). which selectively adsorbs impurities (such as CO, CO2 and CH4) at high pressures. Pure hydrogen is collected and impurities are removed by reducing the pressure.
– Membrane separation: polymer or metal membranes selectively allow the passage of hydrogen and separate it from other gases. This method is efficient in energy consumption. And it can be used for small to medium operations.
2. Adsorption of Carbon Dioxide:
– Chemical adsorption: This process involves the reaction of CO2 with a chemical solvent. The most common solvents are amines. such as monoethanolamine (MEA). CO2 reacts with MEA to form a reversible compound that can then be heated to release pure CO2 and regenerate MEA:
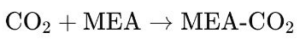
– Physical absorption: In this method, CO2 is absorbed without chemical reaction to a physical solvent (such as Selexol or Rectisol). Physical absorption is more effective at high pressures and is reversible with decreasing pressure.
– Cryogenic separation: cryogenic methods can be used for high purity CO2. The gas mixture is cooled to a very low temperature, where the CO2 liquefies and can be separated from the other gases.
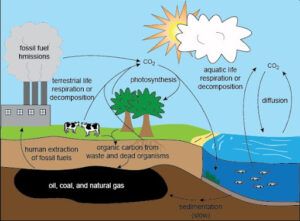
Applications and Importance:
– Hydrogen is vital for industries such as ammonia production (for fertilizers), refining, and as a clean fuel in fuel cells.
– Captured CO2 can be used in enhanced oil recovery (EOR), as a feedstock for chemicals, or sequestered to reduce greenhouse gas emissions. Separation of CO2 from syngas is particularly important in efforts to produce low-carbon or carbon-neutral fuels. These absorption processes are critical to the efficient use of syngas and play an important role in the broader context of energy transfer and carbon management.
Conclusion
Synthesis gas, hydrogen and carbon dioxide adsorption processes are of great importance in the chemical, energy and environmental industries. These processes play a key role in producing clean fuels, chemicals and reducing greenhouse gas emissions.
The production of synthesis gas, hydrogen and carbon dioxide adsorption are processes that play an important role in the transition to a low-carbon and sustainable economy. By developing new technologies and improving the efficiency of processes, it is possible to produce products with added value, reduce greenhouse gas emissions and create new job opportunities.
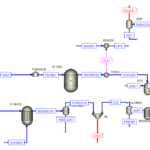
Marjan Petrochemical Methanol Unit was carried out by SANILCO in the following three ways.
1. Full Simulation of Marjan Petrochemical Methanol Unit
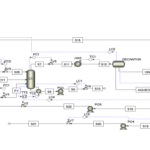
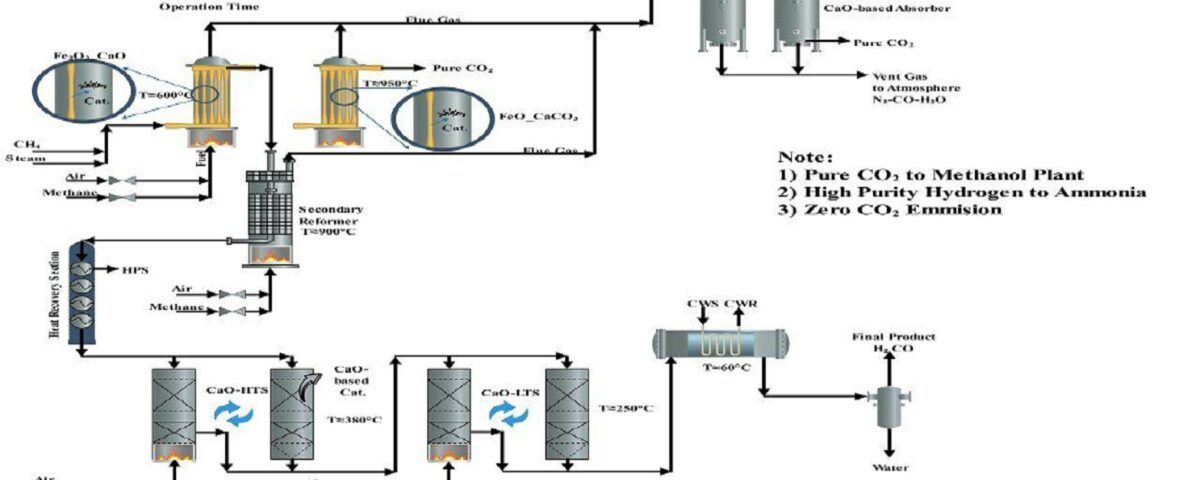
2. Conceptual Design of a New Process to Produce Synthesis Gas, Hydrogen and Adsorb Carbon Dioxide
3. Comparison of Synthesis and Industrial Gas Production Process of Marjan Petrochemical Methanol Unit
Designing The Process of Syngas, H2 and CO2 Adsorption
In this project, the conceptual design of a new process for the production of synthesis gas, hydrogen and carbon dioxide adsorption has been carried out.