Introduction
The reactive distillation unit for acetic acid esters plays a vital role in the chemical industry, significantly contributing to the production of high-value products. This process combines chemical reactions and simultaneous product separation, enabling the production of esters with high purity. The use of advanced technologies and equipment in this system facilitates performance optimization and increased production yields. Fundamental and detailed engineering in the design and implementation of these units aids in precise process analysis, equipment selection, and economic evaluation of the project. Given the growing importance of esters in various industries, including pharmaceuticals, food, and cosmetics, the proper engineering and design of these units is crucial.
In this context, reactive distillation serves as an efficient solution to reduce resource waste and enhance productivity. Therefore, precise analyses and advanced simulations are essential for better understanding the reactions and system dynamics. This allows for the determination of optimal operational parameters, production cost reduction, and improved final product quality.
Thus, effective design and implementation of the reactive distillation unit, along with attention to environmental and economic issues, can herald a more sustainable and successful future in the chemical industry. Therefore, focusing on the technical, economic, and environmental aspects in the fundamental and detailed engineering of these units is of particular importance and can serve as a successful model in the industry.
Reactive Distillation Unit
The engineering design of the reactive distillation unit, as a key process in the production of acetic acid esters, requires consideration of several critical factors. It is essential to choose suitable equipment such as distillation columns, reactors, boilers, and coolers, as their proper selection ensures optimal performance and energy efficiency. Additionally, defining operational parameters like temperature, pressure, and phase behavior of reactants is crucial for achieving the highest conversion rates and product purity.

Utilizing chemical modeling and computational simulations in the design process can assist engineers in predicting system performances and optimizing conditions. Furthermore, safety and environmental considerations must be integrated throughout the design to ensure the process is both economically viable and sustainable. Ultimately, a comprehensive and conceptual design enhances production efficiency while also reducing costs and production time.
Industrial Importance
This unit, due to its use of reactive distillation, can improve reaction yields by eliminating produced water while facilitating the separation of the final product. Designing this process necessitates selecting an appropriate reactor (e.g., CSTR or Batch), optimizing the distillation column design, and determining the conditions for temperature and pressure. Moreover, economic implications and evaluations of operational costs alongside adherence to safety standards and environmental waste management are critical. Overall, given the growing market demand for acetic acid esters, this project has high feasibility and profitability potential.
The industrial significance of producing acetic acid esters is notable due to the extensive applications of these compounds across various industries. Esters serve as solvents, additives, and key raw materials in the production of diverse industrial products such as paints, coatings, fragrances, food products, and pharmaceuticals. With the unique characteristics of esters, including aroma, flavor, and solubility in organic solvents, the demand for them continues to rise. Furthermore, utilizing modern technologies like reactive distillation enhances process efficiency, leading to reduced waste and costs. Considering the growing global market trends and environmental needs, this process holds substantial importance not only economically but also in terms of sustainability.
Process Description
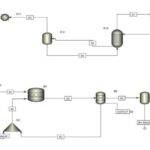
The production process of acetic acid esters through reactive distillation involves the combination of acetic acid with alcohol in a reactive distillation unit, where both chemical reaction and product separation occur simultaneously. In this process, using an appropriate catalyst, acetic acid and alcohol react under controlled temperature and pressure to produce ester and water. The resulting vapor from this reaction is transferred to the distillation column, where distillation principles are applied to separate the ester from water and other byproducts.

Heat and pressure are continuously adjusted to achieve maximum conversion and purity of the final product. Moreover, optimizing process control and management systems can enhance yield and energy savings. This method not only contributes to lowering production costs but also facilitates the efficient use of raw materials and reduces environmental pollution.
Example of Designing and Implementing an Ethyl Acetate Production Plant for Process Analysis and Economic Solutions to Meet Global Demand
This project utilizes various production techniques, with the primary focus on the dehydrogenation of ethanol as the most effective method. Given the global demand for ethyl acetate, projected to reach 4.9 million tons per year, this proposed facility aims to fill a market gap of 2.5 million tons, contributing approximately 4%.
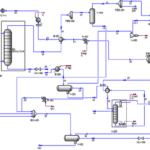
The production process of ethyl acetate, a key chemical in the fragrance industry, involves several critical stages and the use of various equipment. In this project, the production of ethyl acetate through the hydrogenation of ethyl alcohol is examined as the primary method. This process is conducted in a closed multi-tube reactor known as CRV-100, utilizing a copper-chromite catalyst on alumina along with barium chromate. The optimal conditions for this reaction include a temperature of 245.3°C and a pressure of 20 bar.
In the initial stage, ethyl alcohol is converted to ethyl acetate, using a fixed tubular heat exchanger to maintain the heat generated from endothermic reactions. Calculations indicate that the required catalyst weight is approximately 2190.109 kg, which is suitable for the reactor’s capacity.
After production, the product is transferred to a distillation column (T-102) designed to separate ethyl acetate from ethyl alcohol. This column features 16 distillation stages and a height of 9.416 meters. Additionally, two more heat exchangers (E-100 and E-101) are employed to enhance separation efficiency by reducing the temperature before entering the separator.
The economic analysis reveals that this project will be financially sustainable, with an annual revenue of approximately RM 699,209,702 and a payback period of about 2.01 years. Waste management is also critical in this process; hence, treatment systems for liquid waste, gaseous emissions, and spent catalysts have been designed to comply with environmental standards.
Conclusion
Due to various challenges in this field, the process requires meticulous attention to design principles, the selection of appropriate catalysts, operational conditions, and control systems. This process not only provides numerous economic advantages but is also recognized as a sustainable solution through energy consumption optimization and waste reduction.
With advancements in new technologies and engineering innovations, it is expected that the efficiency of this method will increase, leading to significant improvements in ester production. Therefore, research and development in this area can enhance product quality, reduce production costs, and direct the industry toward a more optimized and sustainable future.
Design and Engineering of a 100,000 TPD Acetic Acid Ester Production Unit with Reactive Distillation
In this project, the design and engineering of a reactive distillation unit for acetic acid esters with a capacity of 100,000 TPD have been conducted.