Description
Styrene is a highly important organic chemical that serves as a building block for polymers in the production of a wide range of plastic products. It is a colorless, oily liquid with a sweet, somewhat pungent odor.
Chemical Structure and Properties
Styrene consists of a benzene ring and a vinyl group. This structure gives it unique properties such as high reactivity, insulating properties, transparency, and resistance to chemicals.
Applications
Styrene is used as a raw material in the production of many plastic products, including polystyrene (disposable tableware, insulation), ABS (automotive parts, appliances), unsaturated polyester (composites, resins), and synthetic rubber.
Hazards
Styrene is a chemical substance and can cause irritation and damage if it comes into contact with the skin, eyes, or is inhaled. Long-term inhalation can also have adverse health effects. When working with styrene, protective equipment such as gloves, safety glasses, and masks should be used, and the work environment should be well-ventilated. Due to its unique properties and wide range of applications, the demand for styrene is expected to increase in the future. However, due to environmental and health concerns, efforts are being made to develop alternative polymers with lower environmental impacts.

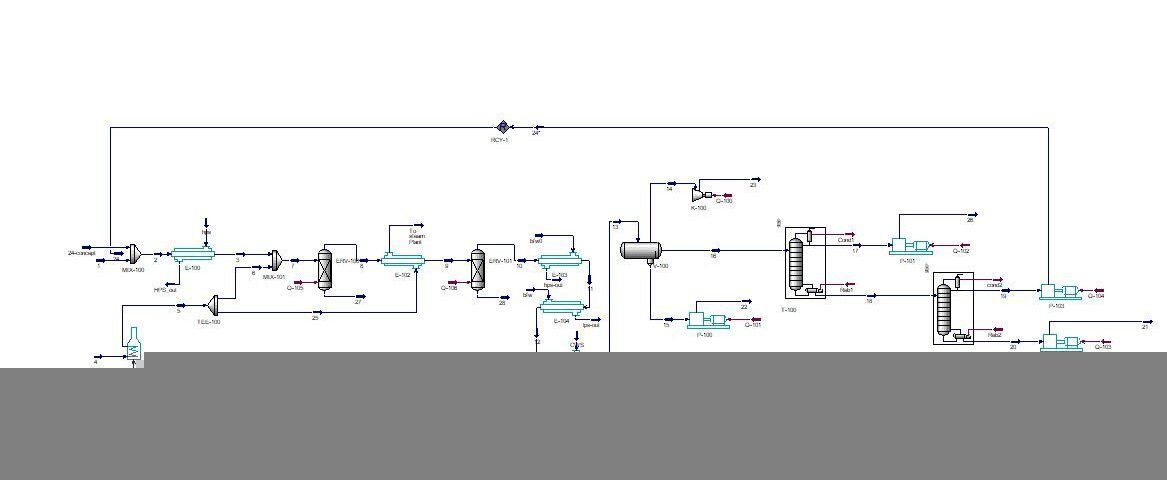
Styrene is one of the most widely used petrochemical products, after ethylene and propylene, it is the third product with high production in the world andThe industrial process of styrene production is based on the dehydrogenation of ethylbenzene, which is carried out at a temperature of nearly 600 degrees Celsius in the vicinity of water vapor. The process has two reaction stages and two separation stages, all of which are simulated with Hysys software.
The simulation from the book is done in a conceptual and detailed way and has a training video and a brief description of the simulation.