Introduction
Gas hydrates are ice-like solid compounds formed when gas molecules (such as methane, ethane, propane, and carbon dioxide) combine with water molecules under specific temperature and pressure conditions. These compounds are abundant in nature, particularly in deep ocean sediments and permafrost regions. Carbon dioxide hydrates, a specific type of gas hydrate, have gained significant attention in recent years due to their potential applications in carbon capture and storage, as well as natural gas recovery.
Formation Mechanism
The formation of gas hydrates is a complex process influenced by various factors, including temperature, pressure, gas type, water composition, and the presence of impurities. In carbon dioxide hydrates, CO2 molecules are trapped within cage-like structures formed by water molecules. This cage-like structure gives hydrates a crystalline appearance.
Carbon Dioxide Hydrate Formation
Natural gas hydrate (NGH) is considered a potential hydrocarbon resource with significant reserves, primarily found in submarine sediments and permafrost. Current research on NGH focuses on exploring its properties and developing production methods. NGH production methods can be broadly categorized into four groups:
- Thermal stimulation to increase formation temperature above the NGH equilibrium temperature.
- Pressure reduction to decrease reservoir pressure below the NGH equilibrium pressure.
- Injection of thermodynamic hydrate inhibitors to disrupt the NGH equilibrium.
- CO2 substitution.
The first three methods are primarily limited by low heat transfer efficiency in the reservoir, which restricts the rate of NGH dissociation. Additionally, these methods rely on the principle of hydrate dissociation, leading to reduced NGH reservoir strength and potential slope instability, seabed damage, and other environmental issues.
Modeling Carbon Dioxide Hydrate Formation with COMSOL
This project involves modeling carbon dioxide hydrate formation using the COMSOL software. The CO2 substitution method offers a dual benefit of energy utilization and greenhouse gas sequestration, but the CH4 substitution rate is relatively low. When an inhibitor is added, CH4 hydrate and CO2 hydrate exhibit different thermodynamic stabilities. In this context, a novel method for NGH production has been proposed and experimentally verified. This new method combines CO2 substitution with thermodynamic hydrate inhibitor technology to accelerate CH4 hydrate dissociation.
A new method for the replacement of CH4 with CO2 in natural gas hydrate production
Simulating the chemical kinetics of CO2-methane exchange in hydrate
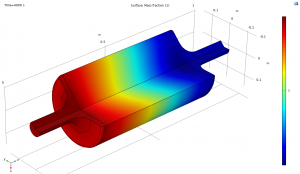
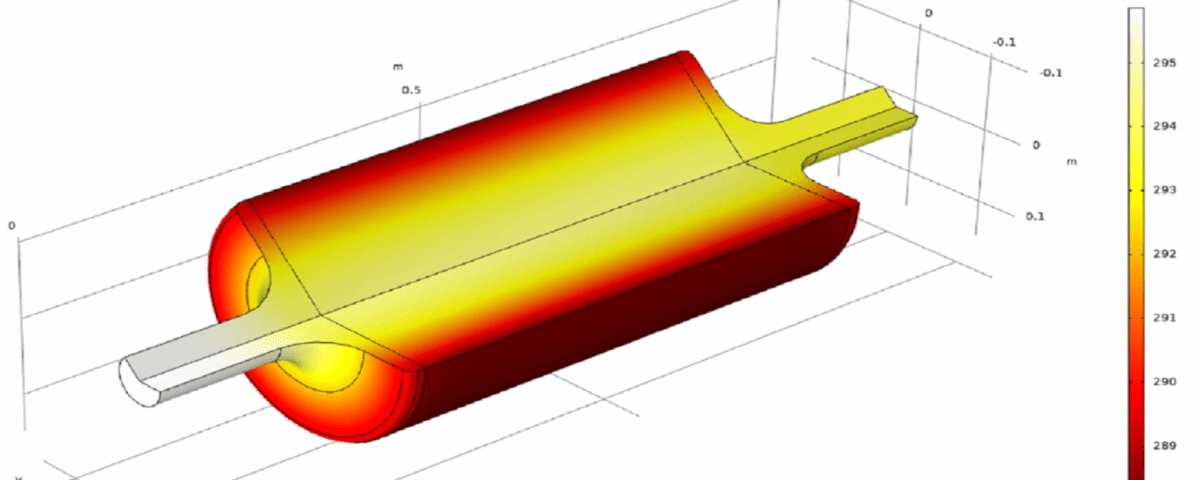
The following images show part of the simulation results.
Mass fraction of CH4
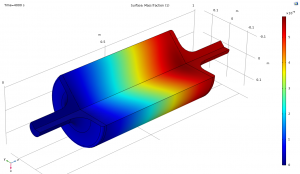
Mass Flow CO2