Description
Cyclohexane has the chemical formula C6H12. which is known by the names of hexahydrobenzene, hexamethylene, hexanaphthene. And its molecular weight is 84.16. This substance is a colorless liquid with a smell similar to ethers, which evaporates easily. And it is insoluble in water and has a lower level of toxicity compared to benzene.
Natural gasoline usually contains about 5-15% cyclohexane and it depends on the type of crude oil from which it was extracted. The complete separation of cyclohexane is difficult due to the presence of hydrocarbons with close boiling points. Therefore, the purity of the obtained cyclohexane is between 75-85%. In general, cycloalkanes are found in large quantities in oil and are known as naphthenes in the oil refining industry. Usually, except for compounds with low molecular mass, such as cyclopentane and cyclohexane, they are not found as separate compounds.
Cycloalkanes can be converted into aromatic hydrocarbons during the reforming reaction. Therefore, they are a great source for the production of valuable aromatics. Cyclohexane has the lowest twist angle and chain among cycloalkanes. And this factor causes the most stability among cycloalkanes. Therefore, compared to other cycloalkanes, less heat is obtained as a result of its combustion.

Cyclohexane is produced by two methods as follows.
Method 1: Production of Cyclohexane From The Distillation of Hydrocarbons and Then Their Purification
Natural gasoline usually contains about 5-15% cyclohexane. and depending on the type of crude oil from which it was extracted. The complete separation of cyclohexane is difficult due to the presence of hydrocarbons with close boiling points, so the obtained cyclohexane purity is between 75-85%. As a result, this method is only applicable in cases where cyclohexane is used as a solvent. Petroleum Philips Company in America produces cyclohexane from natural gasoline.
In order to produce cyclohexane with a purity of more than 85%, the distillation method can be used except for cutting hexane obtained from crude oil. In this process, methylcyclopentane is first separated from the mixture. Then this stream is mixed with Point-End-Low gasoline and the existing cyclohexane is converted to benzene and methylcyclopentane near the catalyst.
From the obtained product, pentanes and solid materials are first separated, and then during the distillation process, a mixture of 90% cyclopentane and 10% benzene is obtained.
This stream, along with cyclopentane obtained from the first distillation, is transformed into its isomer, i.e. cyclohexane, by hydrogen and in the presence of aluminum chloride catalyst. After distilling the output from the isomerization reactor, cyclohexane is obtained with a purity of 98%. It is worth mentioning that some adipic acid production factories use this type of cyclohexane in product production after removing 2% impurities.
Method 2: Cyclohexane Production From Benzene Hydrogenation
About 89% of the companies producing cyclohexane obtain the product during the hydrogenation reaction of benzene as described below.
![]()
In this method, the purity of the product depends on the purity of the benzene and hydrogen feed and the type of catalyst. And the purity of the product can reach 99.99%.
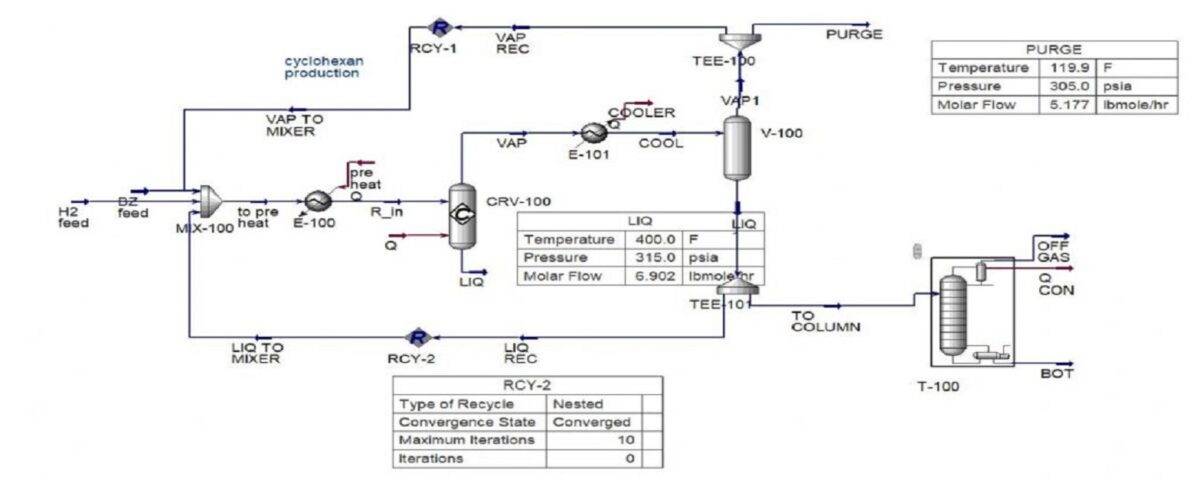
Simulation of Cyclohexane Production Process
In this project, the production process of cyclohexane from benzene is simulated in Aspen Hysys software.