Description
Biodiesel is a type of fuel that is produced by processing and changing the properties of vegetable oils. This fuel was first introduced in the 1970s as an alternative to petroleum diesel, but it was not well received at that time. Nowadays, due to the reduction of oil resources and the increasing need for different types of fuel, the use and production of biodiesel has been given attention and importance.
The size of molecules in biodiesel and petroleum diesel is similar, but these two fuels differ in chemical structure. Biodiesel molecules are almost entirely composed of fatty acid methyl esters that contain olefinic unsaturated compounds. On the other hand, 95% of petroleum diesel (with low sulfur) is saturated hydrocarbons and the remaining 5% are aromatic compounds.
If ethanol is used instead of methanol in biodiesel production, the resulting molecules will be fatty acid ethyl esters. The chemical differences in the structure of these two types of fuel also cause differences in physical properties, which are mentioned below in the form of advantages and disadvantages.

How to Produce Biodiesel
Biodiesel can be produced from vegetable oils, yellow grease, cooking oils or animal fat. This is done by the transesterification process, the products of which are biodiesel and glycerin. Approximately 100 parts (by weight) of oil or fat react with 10 parts of a short chain alcohol (usually methanol) in the presence of a catalyst (usually sodium hydroxide or potassium hydroxide) to finally form 100 parts of biodiesel and 10 parts of glycerin.
Simulation of Biodiesel Separation Process
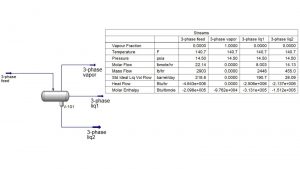
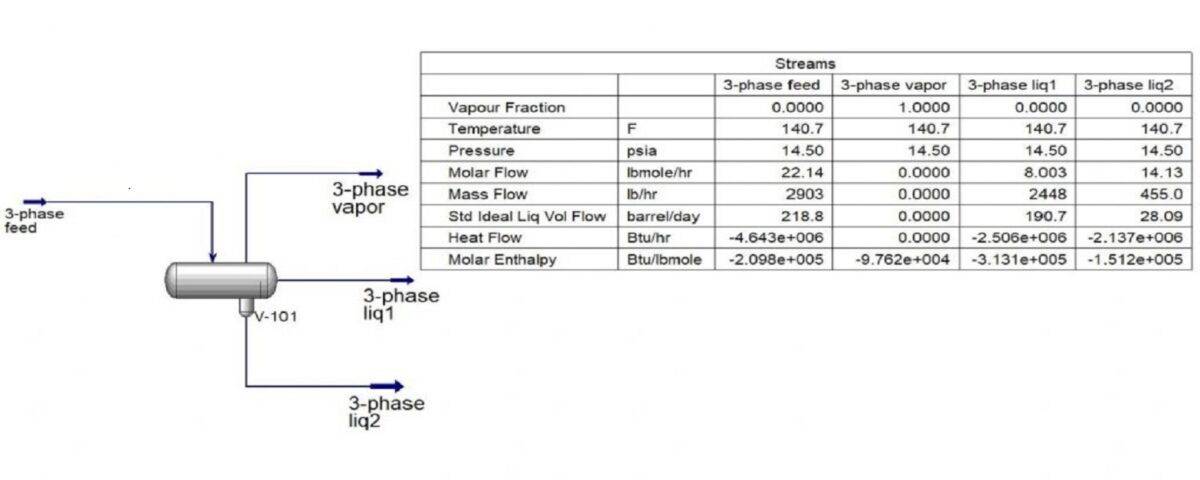
In this project, the simulation of biodiesel separation process in a three-phase separator has been done in Aspen Hysys software.