Optimization of Processes in Oil, Gas and Petrochemical Industries
مهر ۴, ۱۴۰۳
Carrying Out a Professional Comsol Project
مهر ۴, ۱۴۰۳Introduction
With the growth of industries and the need for clean energy, technologies that can provide the production of energy and chemicals with the least amount of pollution have become more important than ever. Meanwhile, electrolyzers, as one of the key technologies, play an important role in creating and expanding industrial processes. These devices perform chemical reactions using electric current and play a significant role especially in the processes of synthesis gas production and removal of carbon dioxide (CO₂) from the atmosphere.

Synthesis gas is used as one of the important bases in the production of fuels and chemicals, and on the other hand, the removal of CO₂ helps to reduce the harmful effects of climate change. In this article, the technology of electrolyzers, synthesis gas production processes, CO₂ removal and their role in industries will be comprehensively reviewed.
Electrolyzer Technology
Electrolyzer technology has become a vital component in various industries due to its extensive capabilities in clean chemical production, energy generation, and pollutant removal. To better understand this technology, we need to go into the details of its operation, its different types, and the recent innovations that have boosted the industrial use of electrolyzers.
Principles of Electrolyzer Operation
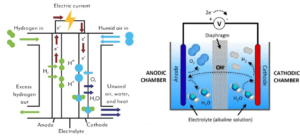
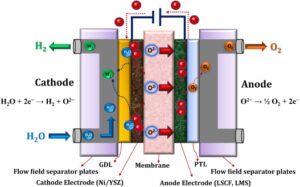
Electrolyzers are devices that use electrical energy to drive chemical reactions. The process of electrolysis occurs when an electric current is passed through an electrolyte (which can be liquid or solid). This electrolyte can include various materials that have the ability to conduct ions. In electrolyzers, there are two electrodes (cathode and anode) on the surface of which chemical reactions take place.
In any electrolysis, two main types of reactions take place:
Oxidation reaction: which occurs at the anode (positive electrode) and involves the loss of electrons by molecules.
Reduction reaction: which occurs at the cathode (negative electrode) and involves receiving electrons by molecules.
These reactions, in combination with each other, break down the primary molecules into their components. For example, in water electrolysis, water molecules (H₂O) are broken down into hydrogen and oxygen.
Types of Electrolyzers
Electrolyzers are divided into three main categories based on the type of electrolyte and operating conditions, each of which has its own uses and advantages in different industries:
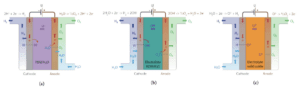
A) Alkaline Electrolyzers
This type of electrolyzer is one of the oldest and most widely used technologies in hydrogen production. In these electrolyzers, alkaline solutions such as sodium hydroxide (NaOH) or potassium hydroxide (KOH) are used as electrolytes. The main advantages of alkaline electrolyzers are:
High efficiency: Due to the simple structure and stable chemical reactions, these types of electrolyzers have high efficiency in hydrogen production.
High durability: the electrodes used in these systems are usually made of corrosion-resistant metals, and as a result, they have a long life.
The main disadvantages of these electrolyzers:
The need for high pressure to produce hydrogen with a larger volume
Slower start than other newer technologies
B) Proton Exchange Membrane (PEM) Electrolyzers
In proton exchange membrane (PEM) electrolyzers, a polymer membrane is used to conduct hydrogen ions (protons). This membrane acts as a solid electrolyte and transfers protons from the cathode to the anode. The advantages of this type of electrolyzer are:
High efficiency: due to the proton conducting membrane, the electrochemical efficiency in this electrolyzer is very high.
Fast response time: PEM electrolyzers have the ability to start faster than alkaline electrolyzers, and this feature is very useful in industrial applications. Compact design: Due to compact design and high operating pressure, these types of electrolyzers are widely used in modern applications such as hydrogen cars.
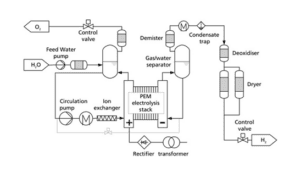
However, the disadvantages of this technology include the high cost of proton membranes and the need to use precious metals as catalysts.
C) Solid Oxide Electrolyzers (SOEC)
Solid oxide electrolyzers are a type of electrolyzer with solid electrolyte that work at high temperatures (more than 700°C). In this type, oxygen ions (O²⁻) pass through the solid electrolyte. The advantages of this technology include:
Very high efficiency: due to working at high temperatures, this type of electrolyzer can directly use the heat lost in industrial processes to provide part of the necessary energy.
Cogeneration: This feature makes SOEC an excellent option for combining with CHP plants.
The main challenges of this technology include very high operating temperatures and the need for high heat resistant materials for the electrolyzer components.
Recent Innovations and Developments
In recent years, according to industrial needs and environmental requirements, many efforts have been made to improve the technology of electrolyzers. Some of these innovations include:
A) Advanced Electrocatalysts
One of the main challenges in electrolyzers is increasing the efficiency of the catalysts used in these processes. Catalysts are substances that increase the rate of electrochemical reactions. In PEM electrolyzers, precious metals such as platinum are used as catalysts, but the high cost of these metals is a great economic challenge.
Recent research shows that the use of catalytic nanomaterials and alternative metal compounds can help improve performance and reduce costs of hydrogen production. These catalysts increase the productivity of electrolytic reactions due to their higher surface area and higher reactivity.
b) Use of Renewable Energies
One of the most important innovations in the field of electrolyzers is connecting them to renewable energy sources such as solar and wind energy. This method helps to produce green hydrogen, which is produced without the use of fossil fuels and is completely compatible with the environment. This hydrogen can be used in various industries to produce synthesis gas, clean fuels, and remove pollutants.
c) High Pressure Electrolysis
Another industrial development is the development of electrolyzers that work at high pressures. These electrolyzers can produce more compact hydrogen, which is very useful for industrial applications such as hydrogen storage and transportation. High-pressure hydrogen production helps reduce compression and storage costs.
The Future of Electrolyzer Technology in Industry
Due to the increasing need for clean energy and environmental solutions, electrolyzers are expected to play a central role in the future of industries. From green hydrogen production to CO₂ removal, this technology can directly help mitigate the effects of climate change and boost clean energy infrastructure. Future plans in this area include optimizing costs, increasing productivity, and developing more advanced electrolyzers for wider industrial applications.
Electrolyzers in Synthesis Gas Production
Electrolyzers are particularly important in the chemical and energy industries, especially in the synthesis gas production and CO₂ removal processes. In recent years, the focus on carbon reduction and energy optimization has made electrolyzers to be recognized as one of the key technologies in the world of clean and sustainable energy. Syngas, which is a combination of hydrogen and carbon monoxide, is used to produce industrial chemicals and fuels, including methanol, liquid hydrocarbons, and ammonia.
Hydrogen Generation for Synthesis Gas: The Role of Electrolyzers in Green Hydrogen

Hydrogen is one of the most important components of synthesis gas and one of the key elements in carbon conversion processes. Using electrolyzers, hydrogen is produced by splitting water into hydrogen and oxygen. The process of electrolysis of water (H₂O), especially using renewable energy such as solar or wind, leads to the production of green hydrogen without the emission of greenhouse gases.
In common technologies for hydrogen production, two types of alkaline electrolyzer and PEM (Proton Exchange Membrane) are mostly used. Alkaline electrolyzers operate using alkaline electrolytes and moderate operating temperatures, and are cheaper and more reliable than PEMs. However, PEMs using proton exchange membranes are more suitable for smaller-scale but high-dynamics applications due to their higher current density and faster reactions.
The use of hydrogen produced by electrolysis is especially important in heavy industries such as steelmaking and ammonia production. The use of green hydrogen in these industries has a significant potential in reducing the consumption of fossil fuels and removing CO₂ from the production cycle.
In addition, various projects at the global level, including projects based on hydrogen production in regions with high renewable energy such as North Africa and the Middle East, are being developed on the basis of water electrolysis, which, if realized, will cause significant changes in the global hydrogen landscape.
Using CO₂ as a Carbon Source for Synthesis Gas Production
In many traditional chemical processes, CO₂ is released into the atmosphere as a waste product and pollutant. However, using CO₂ as a source to produce carbon monoxide (CO) instead of burning fossil fuels can be a good way to produce clean chemicals and fuels. CO₂ electrolyzers, which can decompose CO₂ into CO and oxygen, have the potential to not only prevent CO₂ from being released into the atmosphere, but also turn it into a useful and valuable product.
CO₂ electrolysis process is usually done in two acidic and alkaline states. In the acidic method, proton exchange based membranes are used to transport H⁺ ions from the cathode to the anode where CO₂ is reduced on the catalyst surface. On the other hand, in the alkaline method, CO₂ reacts with OH⁻ ions in an alkaline environment and decomposes into CO and water. The catalysts used in this process play a very important role in increasing CO efficiency and selectivity. For example, the use of gold and silver nanoparticles has significantly increased the efficiency of CO production.
At the industrial level, the use of CO₂ electrolyzers can help reduce dependence on fossil resources and also help realize a circular carbon economy. Many projects worldwide, especially in developed countries, are developing this technology. These projects usually aim to reduce CO₂ emissions and simultaneously produce valuable chemicals from industrial CO₂.
Combining Electrolyzers With Synthesis Gas Production Processes: Production Optimization and Cost Reduction
One of the new and leading ideas in the field of synthesis gas production is the combination of water and CO₂ electrolysis technology to produce a mixture of hydrogen and CO directly. This approach can replace traditional methods such as steam methane reforming and similar processes, which are often associated with high CO₂ emissions and the use of fossil resources.
In these combined methods, electrolyzers with the ability to simultaneously produce H₂ and CO can use cheaper and renewable resources such as water and industrial CO₂ to produce synthesis gas. These processes, also known as “dual electrolysis”, eliminate the need for expensive and complex heat exchangers and provide a significant improvement in terms of investment and operating costs.
Another advantage of this method is the precise control of the ratio of H₂ to CO, which can be optimally adjusted based on process needs. For example, in the production of methanol, the ratio of H₂ to CO must be specifically adjusted to increase the efficiency of the process. The use of electrolyzers in these methods allows rapid change of ratios and greater flexibility in operating conditions, which is a great advantage compared to traditional methods.
Challenges and Opportunities: Development and Progress of Electrolysis Technology
Although electrolyzers are recognized as key technologies in the future of energy and chemical industries, there are still several challenges in the way of widespread application of these technologies. Among these challenges, we can mention the high initial investment costs for the installation and operation of electrolysis units, as well as the operational costs related to the production of electricity from renewable sources.
Another challenge is the need to develop more stable and cheaper catalysts. Currently, catalysts based on precious metals such as platinum and palladium are used in many electrolyzers, which increases costs. Research on alternative catalysts such as carbon nanoparticles, iron and cobalt-based materials, as well as the development of new membrane systems, can overcome these challenges to a large extent.
Finally, one of the biggest opportunities to expand the use of electrolyzers is to reduce the costs of renewable electricity generation and improve energy storage technologies. By reducing the costs of wind and solar energy and developing advanced batteries, it is possible to produce hydrogen and synthesis gas at a lower cost and in a sustainable manner.
Removal of Carbon Dioxide (CO₂) Using Electrolyzers
The removal and recycling of CO₂ from the environment and its reuse as feedstock for the production of chemicals and fuels has become one of the main issues in the chemical and energy industry. CO₂ electrolyzers have the potential to not only help remove CO₂ from the environment, but also convert it into valuable industrial materials.
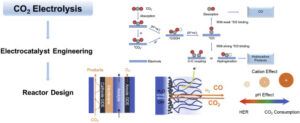
CO₂ Electrolysis Process: Principles and Mechanisms
In the CO₂ electrolysis process, electricity is used to break down CO₂ molecules into CO and oxygen. This process includes electrochemical reactions at the surface of the cathode and anode. At the cathode, CO₂ is converted to CO by receiving electrons (electrochemical reduction). This reaction requires efficient catalysts with high selectivity towards CO production.
Since the CO₂ reduction process in the cathode requires high energy, it is very important to choose the right catalyst to optimize the energy and increase the efficiency of the process. One of the big challenges of this process is reducing the voltage required to activate the reaction.
Catalysts based on precious metals such as gold and silver have so far shown the highest efficiency, but their high costs are a challenge for commercial use. In recent research, copper nanoparticles and inorganic organic materials have also been proposed as alternative catalysts that can reduce costs and have good efficiency.
In the anode, the oxidation reaction of water to oxygen occurs, which is accompanied by the production of protons and electrons. This reaction also helps generate electricity, and some newer designs use advanced electrodes and protective coatings to extend the life of the anodes. Combining this process with energy storage technologies can help in the optimal use of electricity produced from renewable sources.
Applications of CO₂ Electrolyzers in Industry: Production of Clean Fuels and Chemicals
One of the most important applications of CO₂ electrolyzers is the production of clean fuels such as methanol, dimethyl ether (DME), and other hydrocarbons. CO produced through the electrolysis of CO₂ can be directly used as a feedstock in synthetic fuel production processes.
For example, CO and hydrogen produced from electrolyzers can be used in the Fischer-Tropsch synthesis process to produce liquid fuels. In addition to being used as a substitute for fossil fuels, these fuels have higher purity and minimize the emission of harmful pollutants.
Methanol, as one of the most important basic chemicals in industry, has wide applications. This substance is usually produced through natural gas reforming, which is accompanied by the release of CO₂. However, the production of methanol from electrolyzed CO₂ can help reduce carbon dioxide emissions. Since methanol itself can be used as a clean fuel, its importance in the energy and transportation industries is increasing over time.
New Technologies in CO₂ Electrolyzers: Process Optimization and Production Scale Increase
The development of CO₂ electrolyzers on an industrial scale depends on several challenges, including the stability of catalysts, the cost of power generation, and the reduction of operating costs. One of the new approaches in this field is the use of high-current-density electrolyzers, which can produce CO on a larger scale.
These electrolyzers typically work with ion exchange membranes and advanced nanoparticle catalysts, producing higher current densities per unit area that help increase production efficiency. In addition, the development of hybrid systems that combine processes such as artificial photosynthesis with CO₂ electrolysis is another recent innovation. In these systems, sunlight is used as a direct energy source for electrolysis, which reduces the need for external power sources. These technologies are being investigated and developed especially in countries with a lot of renewable resources such as Australia and Southern Europe.
Future Challenges and Opportunities: Sustainable Development and Scalability
Despite the huge potential of CO₂ electrolyzer technology, challenges such as reducing the costs of catalysts and required materials, increasing the lifetime of systems, and integration with renewable power grids still require further research and development. Applying these technologies on an industrial scale requires reducing operating costs and increasing productivity.
One of the important challenges in the future is to combine CO₂ electrolyzer systems with energy storage technologies. Since electricity production from renewable sources may be intermittent (especially in wind and solar power plants), the development of systems that can store excess energy and use it when needed for CO₂ electrolysis is of particular importance. Advanced lithium-ion batteries and hydrogen storage systems will play a key role in this regard.
Application of Eelectrolyzers in Heavy and Petrochemical Industries
“HyNet” Project in England
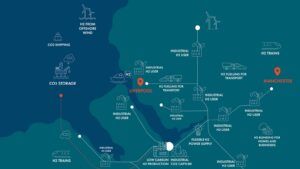
HyNet is a large-scale hydrogen production project using electrolysis on an industrial scale that has been implemented in England. In this project, electric electrolyzers are used to produce clean hydrogen.
This hydrogen is then used in heavy industries such as steelmaking and refineries to replace fossil fuels as an energy source. The project will not only help reduce CO₂ emissions, but is seen as a key step towards achieving greenhouse gas reductions in UK heavy industry.
“Rephyne” Project at the Shell Refinery in Germany
One of the world’s largest industrial electrolyzers, the “Rephyne” project, has been installed at the Shell refinery in Rhineland. This system with a capacity of 10 megawatts continuously produces hydrogen, which is used as feed in refinery processes.
The aim of this project is to reduce natural gas consumption in traditional hydrogenation processes and reduce CO₂ emissions. Refhyne is known as a successful example of the application of electrolyzers in refinery and chemical processes.
“H2Future” Project in Voestalpine Steelworks Austria

One of the largest and most advanced hydrogen electrolysis projects in the world is the H2Future project at the Voestalpine steel plant in Austria. In this project, high-capacity electrolyzers are used to produce green hydrogen, which is used in the steel production process to reduce iron ore and produce steel without CO₂ emissions. The project shows how electrolyzers can drive steelmaking processes that are heavily dependent on fossil fuels to decarbonize.
“Sunfire” Project in Germany: Converting CO₂ Into Fuel
Sunfire is a leading company in the field of converting CO₂ into renewable fuels using electrolysis. Using solid oxide electrolyzers (SOE), the company converts CO₂ and water into hydrogen and carbon monoxide. These materials are then converted into liquid fuels such as kerosene or diesel, which can be used in airplanes and cars. This project has been able to produce clean synthetic fuels using renewable electricity and is known as a successful example of reducing dependence on fossil fuels in the transportation industry.
Yara Green Ammonia Plant in Norway
Yara, one of the world’s largest fertilizer producers, uses electrolyzer technology in Norway to produce green hydrogen, which is used in the ammonia production process. In traditional ammonia production processes, natural gas is used to produce hydrogen, which causes large CO₂ emissions. But in this new project, water electrolysis has been used as a clean alternative to hydrogen production and has significantly reduced greenhouse gas emissions.
“Gigastack” Project in the UK
Gigastack is a project designed to produce green hydrogen using offshore wind energy and large-scale electrolyzers. This project, with a capacity of several gigawatts, plans to inject green hydrogen into the petrochemical, refinery, and power generation industries. The project could significantly contribute to the decarbonisation of the heavy and petrochemical industries in the UK and is seen as one of the most important steps in the transition towards a hydrogen economy.
Air Liquide Company in Canada
Air Liquide, one of the largest producers of industrial gases, has launched a project in Quebec, Canada, that uses the largest electrolyzers to produce green hydrogen. This hydrogen is used as a clean fuel for hydrogen vehicles as well as for industrial applications. This project will help develop hydrogen infrastructure in Canada and reduce CO₂ emissions in various sectors.
Conclusion
CO₂ electrolysis technology is considered as an innovative and sustainable solution for managing and reducing carbon dioxide emissions in various industries. This technology not only helps to produce clean fuels and valuable chemicals, but can also be used as an effective tool in global efforts to deal with climate change and reduce the negative effects of greenhouse gases.
Considering the current challenges, such as the high costs of catalysts and the need to optimize the performance of systems, further development of CO₂ electrolysis technologies is necessary. Investing in scientific research and new technologies, especially in the field of new catalysts and hybrid systems, can help increase the efficiency and scalability of this technology.
In addition, combining CO₂ electrolysis with renewable energy sources and storage technologies can lead to the formation of a sustainable and efficient energy ecosystem. Therefore, special attention should be paid to the development of infrastructure and supporting policies in order to exploit the potential of this technology in the future. Finally, CO₂ electrolysis can be considered as one of the most key tools in achieving the goals of sustainable development and environmental protection in the next century.

