Introduction
Methanol is a colorless liquid, soluble in water, and has a mild alcohol odor. Since 1800, this feedstock has been widely used in the production of industrial chemicals such as formaldehyde, acetic acid, dimethyl terephthalate, methyl methacrylate, and many other chemical compounds. Approximately 60% of methanol consumption is in the production of chemicals, while the remaining 40% is used as fuel. In the fuel sector, methanol has various applications, including direct blending with gasoline, as a feedstock for the synthesis of methyl tert-butyl ether (MTBE, added to gasoline), as a feedstock for the synthesis of dimethyl ether (DME, added to LPG), and in methanol-to-gasoline (MTG) processes.
Methanol Production Methods Although there are different methods for producing methanol, they all generally converge on the following two reactions:
CO + 2H2 → CH3OH
CO2 + 3H2 → CH3OH + H2O
Synthesis Gas and Methanol Production
The primary feedstock used in all production units of this substance is synthesis gas. Synthesis gas is a mixture of carbon monoxide (CO), carbon dioxide (CO2), and hydrogen (H2). It can be produced from various sources and methods, such as natural gas, coal, biomass, and others. Due to its abundance and relatively low cost, natural gas is commonly used today to produce synthesis gas and subsequently this substance. However, the use of coal is also increasing.
Methanol is one of the three most important products in the global chemical industry, and many substances are derived from it. Considering the anticipated shortage of energy resources in the future, the direct use of methanol as a clean fuel or in the production of hydrogen for fuel cells is gaining significant attention.
Methanol Production Process from Natural Gas
In general, the production of this petrochemical product from natural gas involves the following basic steps:
- Desulfurization of natural gas: Removing sulfur compounds to protect downstream catalysts.
- Synthesis gas production: Converting natural gas into a mixture of CO and H2 through processes like steam reforming.
- Synthesis gas compression: Increasing the pressure of the synthesis gas to enhance the reaction rate.
- Synthesis: Converting CO and H2 into methanol using a copper-based catalyst in a reactor.
- Distillation: Separating methanol from other components and impurities.
The synthesis reaction occurs over a copper-based catalyst (CuO/ZnO) known as a synthesis catalyst. This type of catalyst has proven to be effective in both adiabatic and isothermal reactors. The conversion of carbon oxides to methanol is an exothermic process that takes place at high pressures and low temperatures. The synthesis unit typically operates at a pressure of 10-110 bar and a temperature of 200-300 °C.

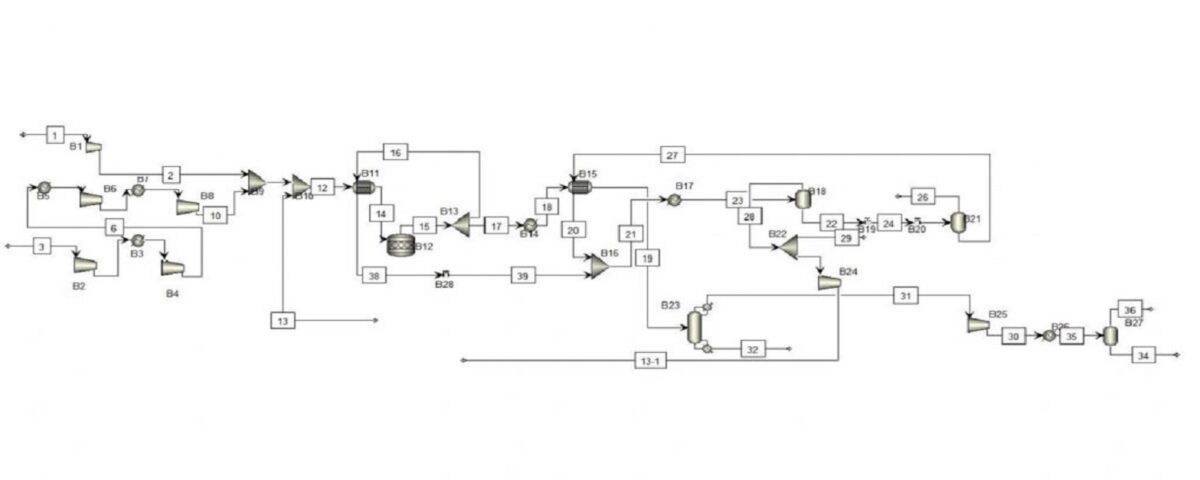
In this project, the conceptual simulation of methanol unit has been done with the help of Aspen Plus software.