Introduction
Tetrahydrofuran (THF) is a colorless, flammable liquid with a distinctive ether-like odor. Classified as a polar ether, THF is a versatile solvent renowned for its ability to dissolve a wide range of substances, including water and various organic solvents. Its solvent properties are attributed to its ability to form hydrogen bonds. The chemical structure of THF consists of a five-membered ring containing four carbon atoms and one oxygen atom.
Applications of Tetrahydrofuran
THF finds extensive applications in various industries due to its excellent solvent properties. Key applications include:
- Polymer production: THF is used in the production of polytetramethylene ether glycol, a polymer used to create elastomers and fibers, enhancing the strength and flexibility of these materials.
- Adhesives: THF is a common solvent in adhesives, particularly for PVC cement.
- Coatings and inks: THF is used in the formulation of coatings, paints, and inks due to its rapid evaporation rate and limited solubility in inorganic compounds.
- Pharmaceuticals: THF is employed as a solvent in various pharmaceutical processes, including Grignard reactions and lithium aluminum hydride reductions.
- Extraction and purification: THF is used as a solvent in extraction and purification processes, such as in reverse-phase liquid chromatography.
- Other applications: THF is also used in the production of leather coatings, vinyl films, and as a solvent in the cleaning of glassware and electronic components.
Simulation of Tetrahydrofuran-Methanol-Water Separation
This project focuses on the separation of a THF-methanol-water mixture using data from the article blow.

The simulation has been done using two conventional extractive distillation tower (CED) and two-effect thermal integration method in an extractive distillation tower (DEHI ED). Both methods are explained in detail in the article. Click on the link below to download the article.
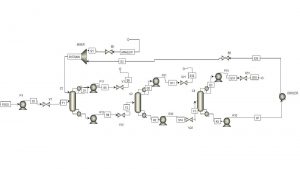
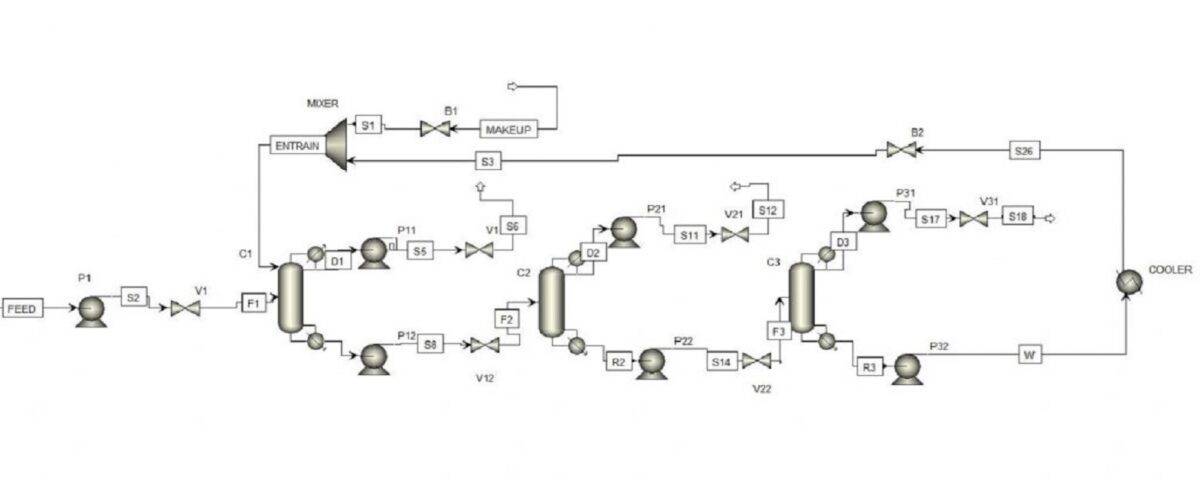
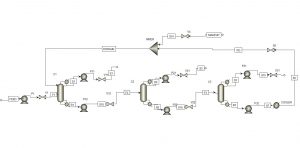
This project was carried out using Aspen Plus software, and the overall simulation view for both methods is shown in the following figures.
Separation of Tetrahydrofuran-Methanol-Water Using the CED Method
Tetrahydrofuran-Methanol-Water Separation with DEHI ED Method