Introduction
Isopropyl alcohol, the smallest member of the secondary alcohol group, is a colorless, volatile, and flammable liquid. With a molecular weight of 60.09 g/mol, it possesses an odor resembling a mixture of ethyl alcohol and acetone. The extensive applications of isopropyl alcohol have made it one of the most significant chemical substances. In this project, after examining the production methods of propanol, we will simulate the production of propanol from propylene.
Isopropyl Alcohol Production Methods
Isopropyl alcohol (isopropanol) can be produced through various methods. However, commercially, it is primarily produced using two methods, both starting with propylene as the feedstock.
Indirect Hydration of Propylene
This method, also known as sulfuric acid hydrolysis, involves the use of concentrated sulfuric acid to absorb propylene. The resulting product is then hydrolyzed to produce isopropyl alcohol. This method has several drawbacks, including high sulfuric acid consumption, severe equipment corrosion, and high energy consumption, limiting its production scale.
Direct Hydration of Propylene
Direct hydration is a newer technology that overcomes the limitations of indirect hydration. It employs active catalysts that are environmentally friendly. This method offers advantages such as high efficiency and low energy consumption. Consequently, it has become a prevalent method for propylene hydration.
Direct hydration can be categorized into three types based on the catalyst:
-
Phosphoric Acid Method (Veba):
- Utilizes phosphoric acid as a catalyst.
- The reaction occurs in the gas phase, and the dissolution of phosphoric acid is suppressed by selecting reaction conditions that retain water in the gas phase.
- This method is suitable for high-purity propylene as a feedstock, achieving high conversion rates per pass.
-
Texaco Method:
- Employs a highly active zeolite or cation exchange resin as a catalyst.
- Due to the catalyst’s high activity and water resistance, the reaction can be conducted at lower temperatures.
-
Heteropolyacid Method (Deshan-Suda):
- A tungsten-based heteropoly acid serves as the catalyst.
- The reaction occurs at high temperature and pressure.
- To enhance propylene conversion, high pressure and a large water-to-olefin ratio are employed, as high temperature is unfavorable for chemical equilibrium.
Among these three methods, the solid acid method offers advantages such as mild reaction conditions, higher propylene conversion, and lower energy consumption compared to the other two methods. As a result, it has gained significant attention. In recent years, many countries and regions have adopted new technologies for isopropyl alcohol production, such as South Korea and Taiwan. However, most isopropyl alcohol production facilities in China still rely on the phosphoric acid and sulfuric acid methods, lagging behind the advanced technologies in foreign countries and unable to meet the demands of economic development.

Simulation of Propanol Production from Propylene
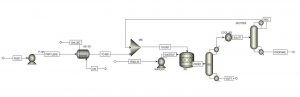
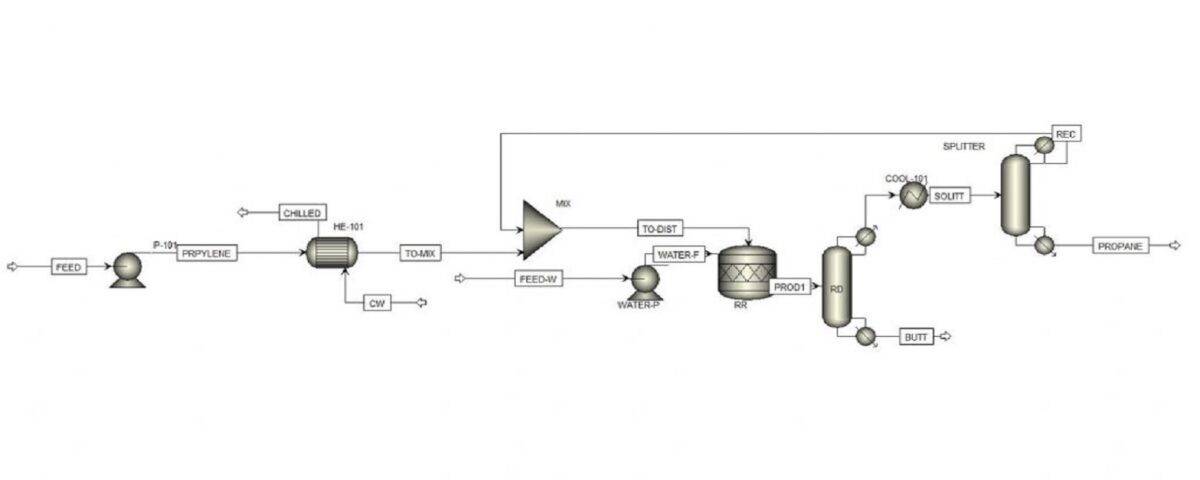
This project involves a process simulation of propanol production from propylene using Aspen Plus software.