Introduction
Vinyl chloride is an organic chlorine compound, Vinyl chloride is also called vinyl chloride monomer. Vinyl chloride is a colorless and industrially important compound because it is used to produce PVC polymer. At room temperature and pressure, vinyl chloride is a gas with a sweet odor and taste. Other names: Vinyl chloride was first produced in 1887 by Justus von Liebig and his student Henri Victor Regnault. They obtained vinyl chloride from the reaction of ethylene dichloride with a solution of potassium hydroxide in ethanol. In 1219, Frans, a German chemist, patented an invention on how to prepare vinyl chloride from acetylene and hydrogen chloride using mercuric chloride as a catalyst.

Vinyl chloride is an intermediate and feedstock for polymerization reactors. In these reactors, vinyl chloride monomer is converted into PVC. Until 1974, vinyl chloride was used in aerosols as a propellant. It was also briefly used as an anesthetic.
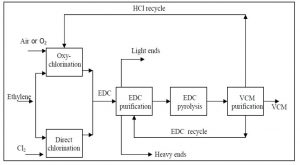
Methods of producing vinyl chloride The production of vinyl chloride involves several reaction steps: Direct Chlorination and the production of vinyl chloride from ethylene dichloride (EDC).
![]()
This reaction has high selectivity ,Thermal Cracking When EDC is heated to 500C at 15-30atm, its molecules are broken into vinyl chloride and hydrochloric acid anhydride.
![]()
This reaction is highly endothermic and is carried out in a thermal heater. Oxychlorination in the new production units of VCM uses the residual hydrochloric acid produced to produce vinyl chloride. For this purpose, they react ethylene, oxygen and hydrogen chloride in the presence of copper chloride 2 catalyst.
![]()
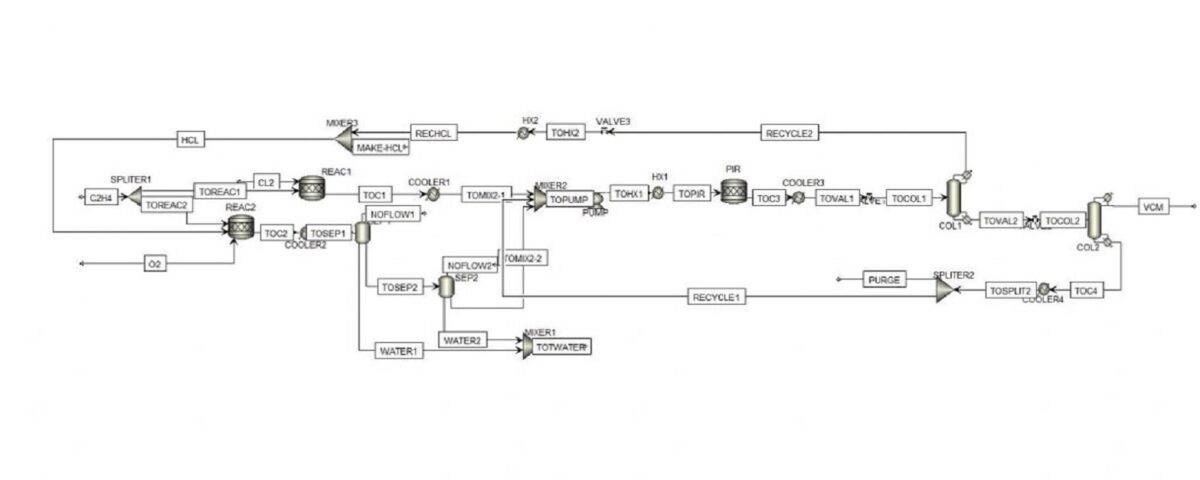
Conceptual Simulation of the Vinyl Chloride Production Unit
In this project, the conceptual simulation of vinyl chloride production unit has been done with Aspen Plus software.