Introduction
Combustion is a process in which a fuel reacts with oxygen to produce heat and light, forming new substances. This exothermic chemical reaction is fundamental to many natural and industrial processes.
Types of Combustion
- Complete combustion: In complete combustion, the fuel reacts completely with oxygen, producing primarily CO₂ and H₂O.
- Incomplete combustion: In incomplete combustion, there is insufficient oxygen for complete combustion, resulting in the formation of byproducts such as carbon monoxide (CO) and soot.
Products of Combustion
The products of combustion depend on the type of fuel and combustion conditions. However, the primary products of complete combustion are:
- Carbon dioxide (CO₂): A colorless, odorless gas produced by the complete combustion of carbon.
- Water (H₂O): Produced in the form of water vapor.
- Energy: Released in the form of heat and light.
Combustion Modeling and Nonlinear Optimization with MATLAB
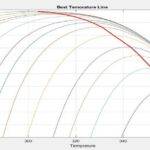
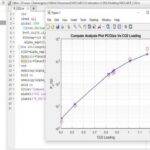
Mathematical formulation and modeling of combustion processes serve as a crucial tool for comprehending this phenomenon. Determining the equilibrium temperature and composition often constitutes the initial step in calculating combustion characteristics. Various techniques exist for simulating combustion processes.
In this project, a fundamental model based on minimizing the Gibbs free energy is proposed for simulating combustion processes. A nonlinear mathematical optimization, grounded in Lagrange multipliers, has been developed and solved using a quasi-Newton method implemented within the MathCAD environment. The influence of various parameters, such as initial temperature, pressure, and equivalence ratio, on the equilibrium temperature and composition has been investigated. This project has been conducted utilizing information from the following article.

Click on the link below to download the article.
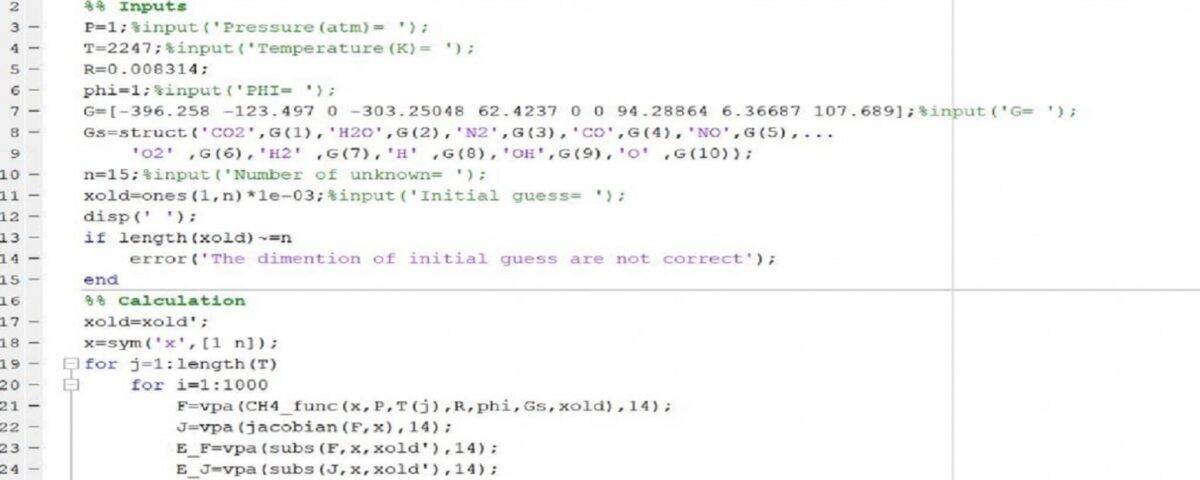
In this project, 14 equations with 14 unknowns have been solved in MATLAB software. Also, the project has an educational video.