Introduction
Air separation refers to the process of separating atmospheric air into its primary components, such as oxygen, nitrogen, argon, and other noble gases. Due to its extensive applications, oxygen ranks third in terms of production volume among all chemicals. It is a crucial element in numerous industrial processes. Some of its major applications include the steel industry, metallurgy, non-ferrous metal production, welding, metal cutting, cement, ceramics, petroleum refining, and papermaking.
The most common method for air separation is cryogenic distillation. However, non-cryogenic methods like pressure swing adsorption (PSA), chemical processes, and membrane separation are also used in commercial units to separate individual components from air. High-purity oxygen, nitrogen, and argon, essential for semiconductor manufacturing, can be produced using the cryogenic method.
Primary Air Separation Methods
- Pressure Swing Adsorption (PSA): This method involves adsorbing gases onto a solid desiccant in a pressurized vessel.
- Membrane Separation: This method uses prefabricated membranes in a pressurized vessel for gas separation.
- Cryogenic Air Separation: This method was pioneered by German scientist Carl von Linde. Despite its high initial investment and energy consumption, it is widely used in projects requiring large-scale production of industrial gases due to its high capacity and product purity. The achievable purity in this system can reach 99.999%.
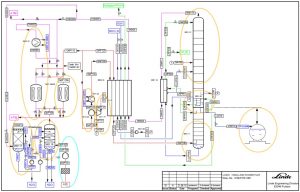
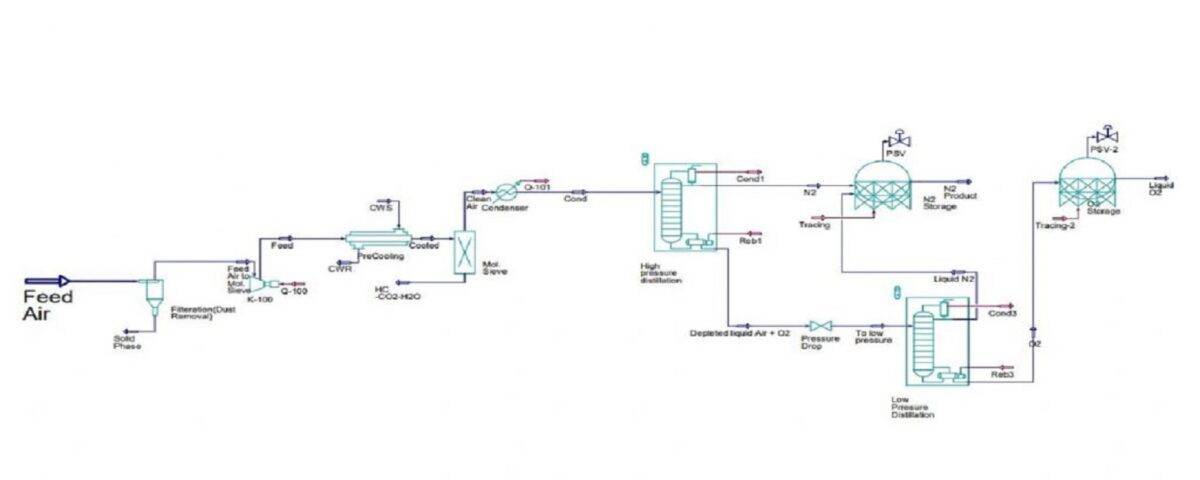
Stages in a Cryogenic ASU
- Air Compression: Air is compressed to a defined pressure (usually between 6-10 bar) using multi-stage turbo compressors equipped with internal coolers.
- Compressed Air Purification: A filtration and drying package removes carbon dioxide, water vapor, and hydrocarbons from the air using molecular sieves. The cooling process is typically achieved using a cooling water system and an evaporative cooler with a nitrogen purge.
- Low-Temperature Heat Transfer: The air is further cooled below its dew point in heat exchangers using excess nitrogen gas from the cold box.
- High Pressure and Low Temperature Generation: The air stream is further compressed by boosters and then cooled to a very low temperature using expansion turbines. This leads to the initial liquefaction of air for entry into the distillation column.
- Separation of Primary Products: The extremely cold air is transferred to the distillation column. As it rises in the column, it cools further and physically separates into its components, forming a liquid on the column trays. Oxygen, with a higher boiling point, condenses first, followed by nitrogen with a lower boiling point.
- Nitrogen gas collects at the top of the column, while liquid oxygen collects at the bottom.
- Pure nitrogen gas is liquefied in a condenser.
- Pure oxygen is collected from the bottom of the low-pressure column and reboiler.
- Liquid nitrogen provides a reflux for the pressure column and is supercooled for the low-pressure column.
- Further separation occurs in the low-pressure column, with pure oxygen exiting from the bottom and pure nitrogen from the top.
This process offers a highly efficient and reliable method for producing large quantities of high-purity oxygen, nitrogen, and other gases for various industrial applications.

Simulation of a Cryogenic Air Separation Unit
This project presents a simulation study of an air separation unit (ASU) ,The ASU employs cryogenic distillation to produce high-purity nitrogen and oxygen from atmospheric air. The process was simulated using Aspen HYSYS, and a comprehensive report is available.