Description
Acrylonitrile is an organic compound with the chemical formula CH₂=CHCN. This compound is a volatile, colorless liquid, though commercial grades may contain impurities that can give it a yellowish hue. Its molecular structure comprises a vinyl group bonded to a nitrile group, making it a key monomer for producing widely-used plastics like polyacrylonitrile. In lower doses, acrylonitrile is toxic and highly reactive.
Commercially, acrylonitrile is produced through the ammoxidation of propylene. In this process, propylene, ammonia, and air react in the presence of a catalyst within a fluidized bed. Acrylonitrile is initially used as a co-monomer in producing acrylic and modacrylic fibers. Its applications include producing plastic materials, surface coatings, nitrile elastomers, protective resins, and adhesives. It also serves as a chemical intermediate in synthesizing various antioxidants, pharmaceuticals, dyes, and active agents. Previously, acrylonitrile was used as a disinfectant for food products, flour mills, and bakery equipment.
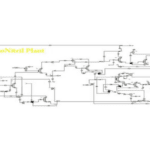
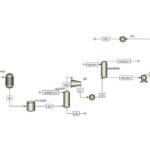
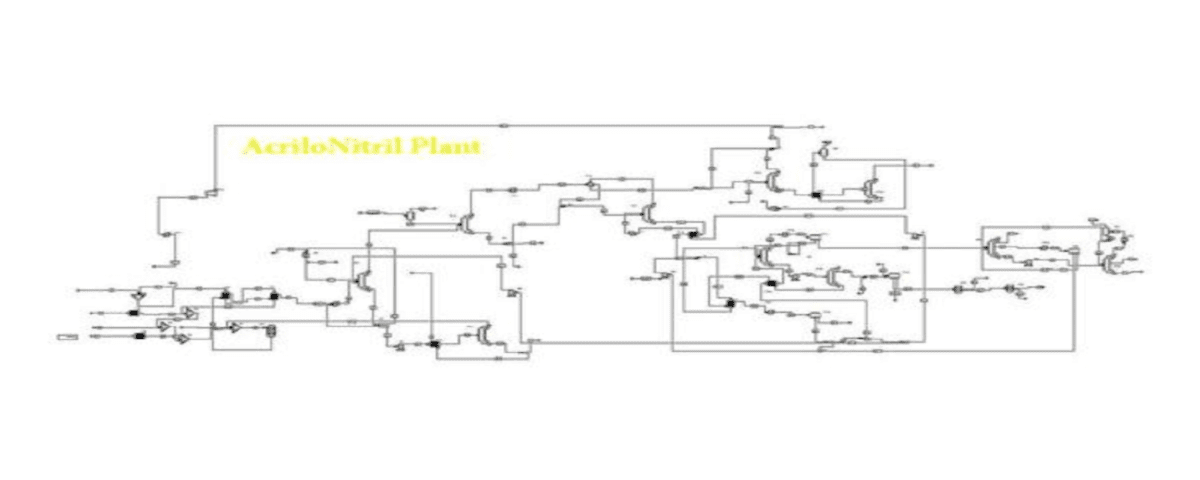
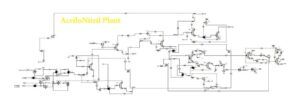
In this project, the acrylonitrile production process from ethylene has been simulated based on process flow diagrams from the SRI Petrochemical Library and using Aspen Plus v10 software.
Additional information about this project is available in the link below.
Available Files: SRI Process Flow Diagrams – Simulation Files