Introduction
The majority of CO2 separation techniques involve physical or chemical absorption of CO2 into a suitable solvent. Due to the strong dependence of physical absorption efficiency on the feed gas CO2 concentration, and the significant decrease in absorption rate with decreasing CO2 content, this method is ineffective for separating CO2 from air where concentrations are typically below a few percent. Consequently, chemical absorption of atmospheric CO2 using amine solutions offers superior efficiency and performance.
This project simulates and analyzes the absorption of CO2 using amine solutions, a common approach for chemical CO2 absorption. Additionally, the study investigates various promoters that enhance the separation of CO2 from air using amine solutions in different absorption towers. The influence of these promoters on the process is evaluated. The project’s primary focus is on removing carbon dioxide from air, and the impact of ionic materials on amine-based CO2 absorption is analyzed through simulation.

Ionic Liquids
An ionic liquid, abbreviated as IL, is a salt in the liquid state. Some texts define ionic liquids as salts with melting points below 100 degrees Celsius (212 degrees Fahrenheit). While ordinary liquids like water and gasoline are primarily composed of electrically neutral molecules, ionic liquid molecules consist largely of ions and short-lived ion pairs.
Ionic liquids have many potential applications. They are very powerful solvents and have high electrical conductivity, making them an excellent choice for use in batteries. Since they have a very low vapor pressure, they are also used as sealants.
Carbon Dioxide Simulation of Capture Using Ionic Liquids
The carbon dioxide capture process from a gas mixture consists of two main parts:
Absorption
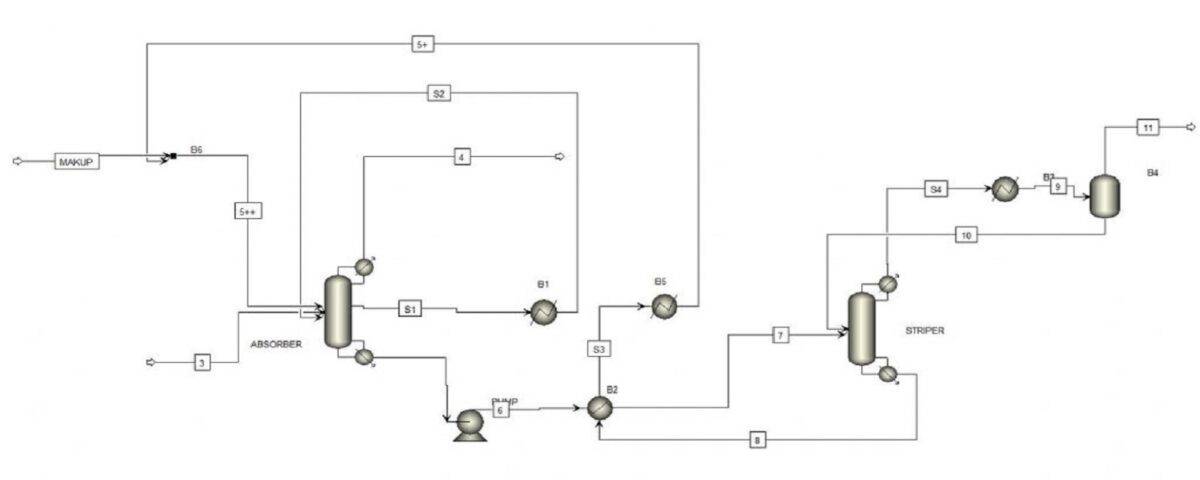
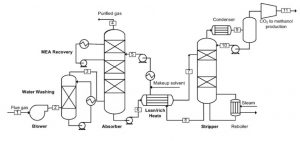
This part consists of an absorption tower where the gas mixture containing carbon dioxide enters from the bottom and the absorbent, which in this case is a mixture of MEA and bmim BF4 dissolved in water, enters the tower. After the absorbent and gas mixture come into contact, due to the high solubility of carbon dioxide in the absorbent, this gas dissolves in the absorbent and exits the tower from the bottom with it. The rest of the gas mixture also exits the tower from the top and enters the environment. To prevent the loss of solvent with the exit gases and to control the tower temperature, a side stream is removed from the top of the tower and re-enters the tower after cooling.
Solvent (Absorbent) Regeneration
In this section, the solvent containing a large amount of carbon dioxide enters a stripper tower. Carbon dioxide gas along with a certain amount of water exits the tower from the top, and the regenerated solvent exits the tower from the bottom, The carbon dioxide gas, after cooling and separating the accompanying water, enters the next stages of the process for further processing. The regenerated solvent is also sent to the absorption section after cooling. The following figure shows a general overview of the process.
In this project, the carbon dioxide absorption process is simulated with the help of ionic liquids with Aspen Plus software. This project is based on the article of Huang et al. (2014) and was carried out using Aspen Plus software. First, using the relationships in the article, the physical and chemical properties of ionic solutions have been introduced to Aspen Plus software. Then the simulation of the corresponding unit is done with this software. It should be noted that all the properties of the ionic liquid in the article have been included in the simulation and we have updated the database for Aspen Plus. Some of the final results of the simulation and the article are as follows:

Also, the project has a full report and an educational video.