Introduction
In recent years, various processes have been employed in the hydrogen industry. One of the most common methods for hydrogen production is natural gas reforming (NG), which is conducted in extremely long plug flow reactors (PFRs) under high temperature and pressure conditions. This project aims to investigate the impact of thermodynamic parameters on hydrogen conversion and the required number of reactors (or reactor length).
General Steps in the Methane Reforming Process
- Preheating: Natural gas and steam are preheated before entering the reformer to reach the reaction temperature.
- Reforming: The mixture of natural gas and steam is passed over a nickel catalyst in the reformer. In this step, the primary reforming reaction occurs, producing hydrogen and carbon monoxide.
- Shift Reaction: The carbon monoxide produced in the previous step reacts with additional steam to produce hydrogen and carbon dioxide. This reaction is known as the water-gas shift reaction.
- Purification: The gas from the shift reaction contains a mixture of hydrogen, carbon dioxide, carbon monoxide, and other impurities.To produce high-purity hydrogen, the gas must be purified. Various methods exist for hydrogen purification, including adsorption, membrane separation, and cryogenic distillation.
Chemical Reactions in Methane Reforming
Primary Reforming Reaction
CH₄ + H₂O → CO + 3H₂
Water-Gas Shift Reaction
CO + H₂O → CO₂ + H₂
Factors Affecting Process Efficiency
- Catalyst Type: The selection of a suitable catalyst significantly impacts reaction rate, selectivity, and reactor lifetime.
- Temperature and Pressure: Increasing temperature and decreasing pressure generally enhance reaction rate and overall efficiency.
- Steam-to-Carbon Ratio: The steam-to-carbon ratio in the feed mixture influences the composition of product gases and the extent of methane conversion.
- Space Velocity: The speed at which the reaction mixture passes through the catalyst bed affects conversion and selectivity.
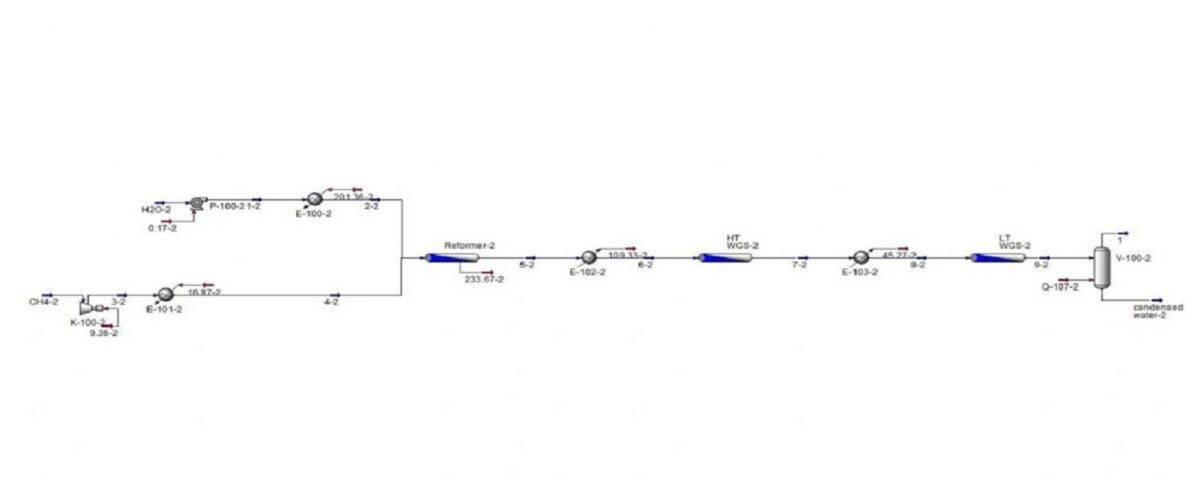
Simulation of the Hydrogen Production Unit of Methane Reforming
This project involves simulating a reforming unit under three different operating conditions using Aspen Plus and HYSYS software.
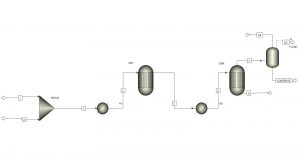
File 1: Aspen Simulation 1 which includes two reactors and a separator.
 File 2: simulation of Aspen No. 2, which has 3 reactors and a separator.
File 2: simulation of Aspen No. 2, which has 3 reactors and a separator.