Description
The need to know the most optimal process is one of the most important issues that is accepted by all designers in the world today. Due to the progress of science and the creation of new processes in order to produce various products from different raw materials with limited capital, this project is an attempt. that among the possible processes and product production methods, the optimal process is selected and checked. In this way, the effects of design variables, including reactor volume and the sequence of distillation towers, and the return flow rate and so on. . . It will help in economic calculations on product production and costs of each.
The reviews given in the upcoming chapters include economic topics and potentials at different levels of design and. . is to minimize operating costs. Because this product may be used as a reactive substance in many processes, the issue of pollution is also raised to prevent the production of a product with pollution that is harmful to the environment.

Product Introduction
The main product investigated in this project is monoethylene glycol, and side products include diethylene glycol and triethylene glycol.
Monoethylene Glycol
Monoethylene glycol is an organic compound with the chemical formula (CH2OH)2, which is a colorless and cloudy liquid with a sweet smell. It is highly miscible in water and has acceptable miscibility in other light alcohols such as methanol, ethanol, propanol, etc.
Monoethylene Glycol Production Process
In this process, monoethylene glycol is produced by the reaction of ethylene oxide with a sufficient amount of water during an exothermic reaction. The reaction is carried out in the aqueous phase. And while producing monoethylene glycol, side products such as diethylene oxide and triethylene oxide are produced by the re-reaction of monoethylene glycol with ethylene oxide. In order to have better selectivity of monoethylene glycol, it is better to enter ethylene oxide and water at a mass ratio of 1:7 into the reactor. Also, to start the reaction, a temperature of 150-200 C and a pressure of 15-20 bar are necessary. The reactions of hydration of ethylene oxide are as follows.
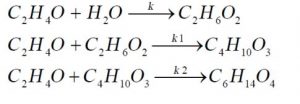
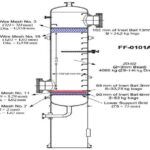
Conceptual Design and Economic Analysis of MEG Unit
The main feed of the unit is ethylene oxide. which is then compressed by the C-1 compressor with water and return flow from the evaporators which contains water and monoethylene glycol. It enters the E-1 converter until it is preheated and reaches the reaction temperature, and then it enters the reactor.
Since the reaction is exothermic, the output of the reactor is at a higher temperature. Since for proper selectivity of the reaction, the mass ratio of water is 6 times that of ethylene oxide, the output products of the reactor are dry with a large amount of water, so there is a three-stage evaporator after the reactor to separate some of the water in the flow and enter the reactor.
After the evaporators, the T-1 distillation tower is located in order to separate the residual water in the flow. After water separation, the flow of products enters T-2 and T-3 towers to separate monoethylene glycol, diethylene glycol, and triethylene glycol, respectively. and enter storage tanks as a product. The process diagram simulated with ASPEN PLUS software can be seen in the figure below.
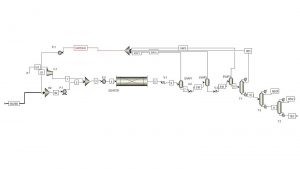
Conceptual design and economic analysis of monoethylene glycol unit has been done in Aspen Plus software. Also, this project has a complete report.