Introduction
Sulfuric acid, as one of the most important and widely used chemicals in the industry, plays a key role in various production processes. This compound with the chemical formula H₂SO₄, because of its special characteristics. Including high acid strength and wide solubility, in various industries such as petrochemical, mineral processing, chemical fertilizer production. Pharmaceuticals and water purification are used. The importance of sulfuric acid is so much that the amount of its production and consumption is considered a suitable criterion for measuring the industrial growth of a country.
Sulfuric acid production unit is usually based on the process of sulfur vapor recovery and its oxidation with the help of catalytic oxidation. This process is widely applied in the chemical industry due to its high productivity and reduced pollution, especially compared to traditional methods. In this regard, design goals, optimal management of resources, and quality control in the production of this material are of particular importance.
Description
Sulfuric acid is produced by two methods: Sulfur burner and Smelter. In Iran, due to easy access to sulfur and preparation of sulfur, the first method is widely used.
Production Steps of Sulfuric Acid
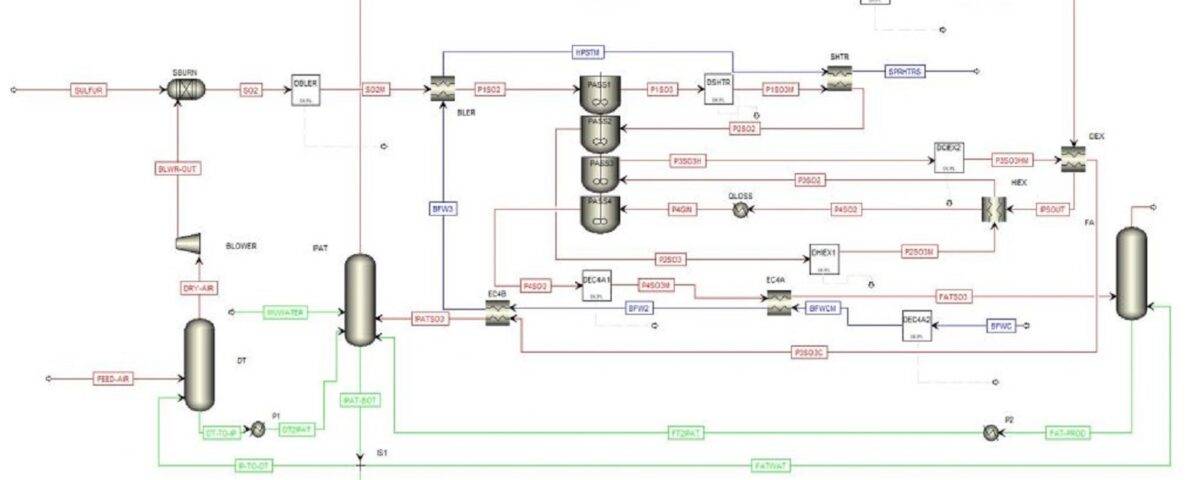
1. The gas flow along with the input feed to the acid production process first enters the drying tower. After that, it turns into sulfur (SO2) by burning sulfur along with the required oxygen.
2. In the next step of the process, the gas stream of sulfur dioxide enters the converter tower to be converted into sulfur trioxide. In the presence of the vanadium pentoxide catalyst, this substance (SO3) is formed.
3. The gas stream is condensed in the Oleum Tower. Then, the sulfur trioxide liquid along with its excess gas is transferred to the Initial Absorption Tower for initial absorption and according to the key equipment inside the tower including Candle Filters, Demister Pads Support packings (Packing Support Grad), packings and the presence of powdered water by atomizing nozzles provide a suitable substrate for initial absorption.
All the excess materials in the process, which due to the size of the particles and the insignificant weight, are not able to be converted into liquid sulfuric acid, are transferred to the final absorption tower (Final Absorption Tower). The key equipment of the final absorption tower is similar to the initial absorption tower, but in some technical and model cases, they are different, which is due to the inability to absorb smaller particles and changes in the process conditions after passing through the initial absorption.

In this project, sulfuric acid production unit is simulated using Aspen Plus software. This project has a complete report and has been written and reviewed in the Acid Synthesis Reactor section, manual calculations of mass and energy balance, and will be sent along with the available diagrams.