Introduction
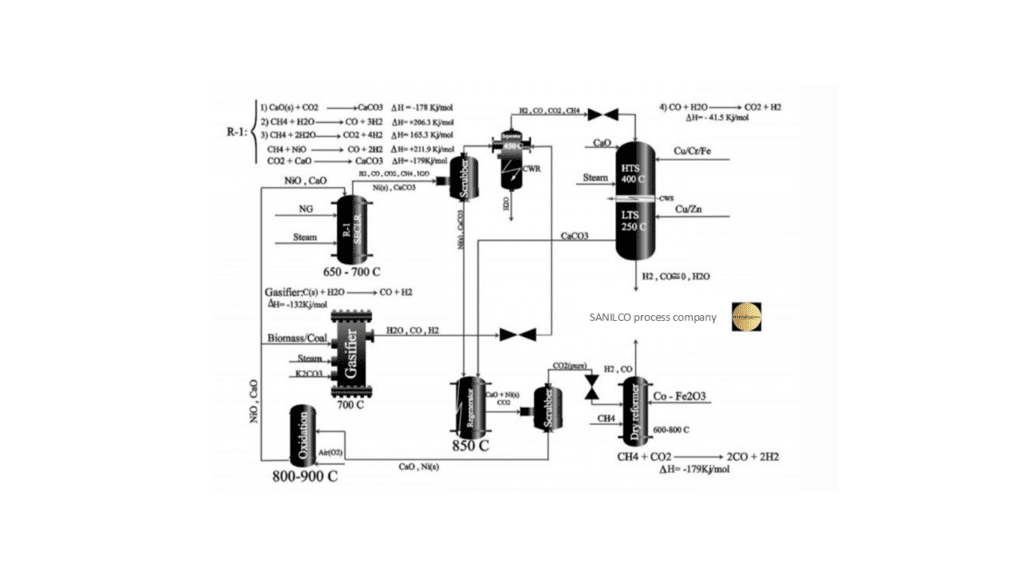
The primary feedstock for ammonia production units consists of sweet gases (primarily methane) and atmospheric nitrogen. Methane, when mixed with steam and subjected to a reaction in the primary reformer, is converted into hydrogen, carbon monoxide (CO), and carbon dioxide (CO2). The reforming reaction is completed by injecting air into the secondary reformer, simultaneously introducing the nitrogen required for ammonia synthesis. The carbon monoxide present in the process gas is subsequently converted to CO2 in subsequent stages, and the resulting mixture is directed to the CO2 absorption section for purification. Carbon dioxide, a byproduct of ammonia synthesis units, is sent to urea production units after separation.
Reforming Reactions
A mixture of nitrogen and hydrogen gas, commonly known as synthesis gas, is compressed and then, under specific temperature and pressure conditions in the presence of a catalyst within a reactor, synthesized into ammonia. The majority of the produced ammonia is utilized in the production of urea and diammonium phosphate fertilizers within the complex. Excess ammonia, beyond the required quantity, is supplied to the international market.
Designing and Simulating a New Process to Produce Synthesis Gas
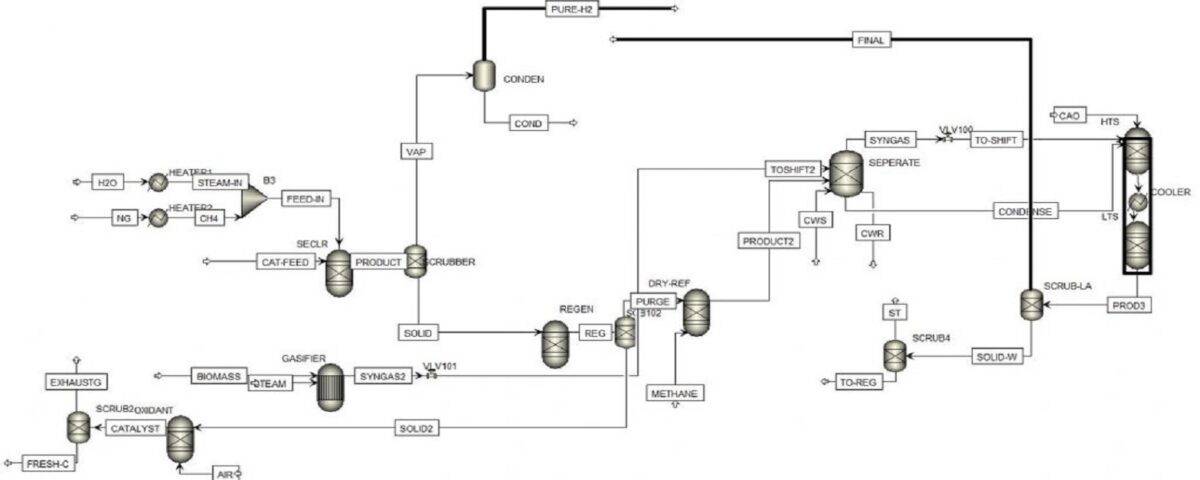
In this project, the design and simulation of a new process for the production of synthesis gas has been carried out with Aspen Plus software and The figure below shows the process PFD.