Description
Ammonium bicarbonate (commonly known as ammonium hydrogen carbonate) is a mild mineral compound and a common reagent in industrial and research methods. This substance is volatile in solution and releases ammonia and CO2.
This property causes that ammonium bicarbonate can be used as a suitable buffer for applications such as lyophilization and matrix-assisted laser ablation. Ammonium bicarbonate is used as baking soda powder, in some food processing applications, in cough syrups, and as an antacid. It is also used in chemical laboratories as a fertilizer, PH buffer and reagent in plants, and in industry it is used in the manufacture of paints, pharmaceuticals, catalysts, ceramics, fireproof materials, plastics and other products.

Chemical Properties of Ammonium Bicarbonate
Ammonia bicarbonate is similar to white monoclinic or orthorhombic white, it is soluble in water, but it does not dissolve in ethanol, carbon disulfide and concentrated ammonia. After dissolving in water, it turns into a slightly alkaline solution and is insoluble in most organic solvents.
Ammonium Bicarbonate Production Method
To produce this substance, compressed carbon dioxide must be sent into concentrated ammonia and put under carbon dioxide pressure and cooling applied at the same time. Following centrifugal separation and dehydration to obtain the final product, crystal precipitation occurs. After purification, it should be dissolved in water and add ethanol to crystallize again.
Carbonization method: After absorbing ammonia by water, carbon dioxide can be applied for decarbonization, followed by separation and drying to produce ammonium bicarbonate.
Applications of Ammonium Bicarbonate
Ammonium Bicarbonate is a white powder food soluble in water that increases its solubility with increasing temperature. It significantly improves the color and volume of bread. In addition, it can act as a buffering agent. Moisture is applied like biscuits. Creating a porous and crispy structure, especially in crackers, biscuits and sweets, is one of the advantages and users of ammonium bicarbonate.
Ammonium bicarbonate is a dough enhancer, a compounding agent, a ph control agent and a buffer. In buffer applications such as lyophilization and desorption, laser absorption into the matrix is used. Ammonium bicarbonate is used as a nitrogen fertilizer, which is used for all types of soil; It can simultaneously supply ammonium nitrogen and carbon dioxide needed for crop growth. This material contains little nitrogen and is easy to prepare.
This substance can promote product growth and product photosynthesis. It causes the growth of seedlings and the growth of leaves. Ammonium bicarbonate can be used as a senior food fermentation agent. Its combination with sodium bicarbonate can be used as a raw material for bread, biscuits and pancakes.
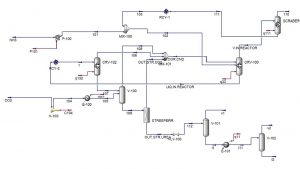
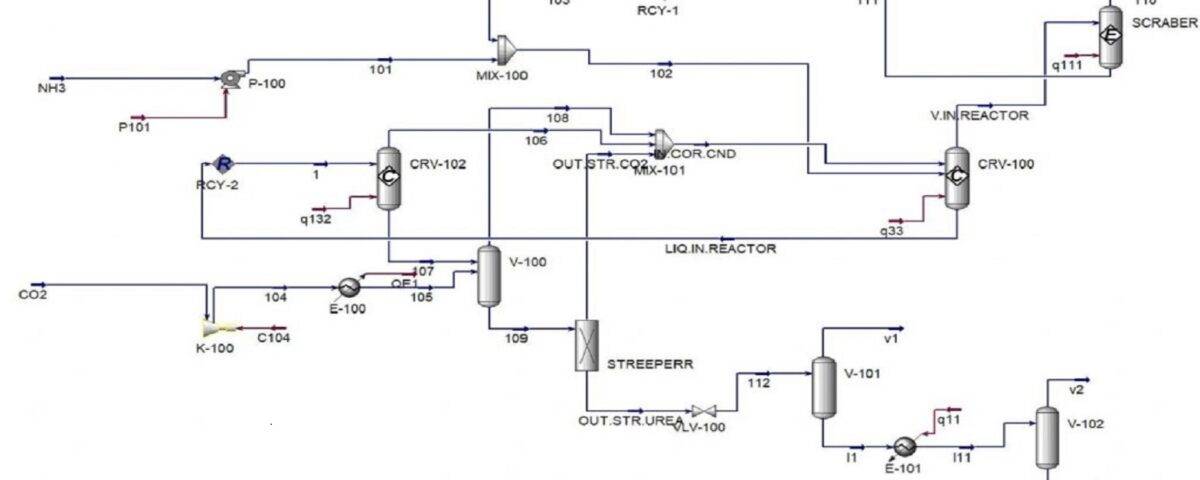
Aspen Hysys Simulation of Ammonium Bicarbonate Process (1)
In this project, the simulation of ammonium bicarbonate production process has been done in Aspen Hysys software.