Description
Batch distillation is a common method for separating mixtures. However, azeotropic mixtures present a unique challenge as their components cannot be separated through simple distillation due to the formation of a constant-boiling mixture. To overcome this, various methods such as the addition of entrainer agents are employed. These agents alter the thermodynamic properties of the mixture, shifting the azeotropic point or eliminating it altogether.
Azeotropic Distillation
Azeotropic distillation is commonly used when the boiling points of the mixture’s components are close. The separation of the initial mixture is facilitated by adding a specific solvent that forms an azeotrope with one of the key components. This azeotrope becomes the product of the distillation or the residue from the column, and the solvent is subsequently separated from the key component. Often, the added substance forms a low-boiling azeotrope, known as an azeotropic breaker. The azeotrope frequently includes components from the feed, but the ratio of key components to other feed components is significantly different and usually higher.
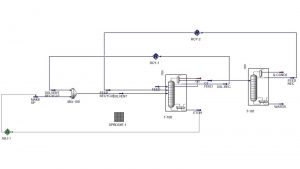
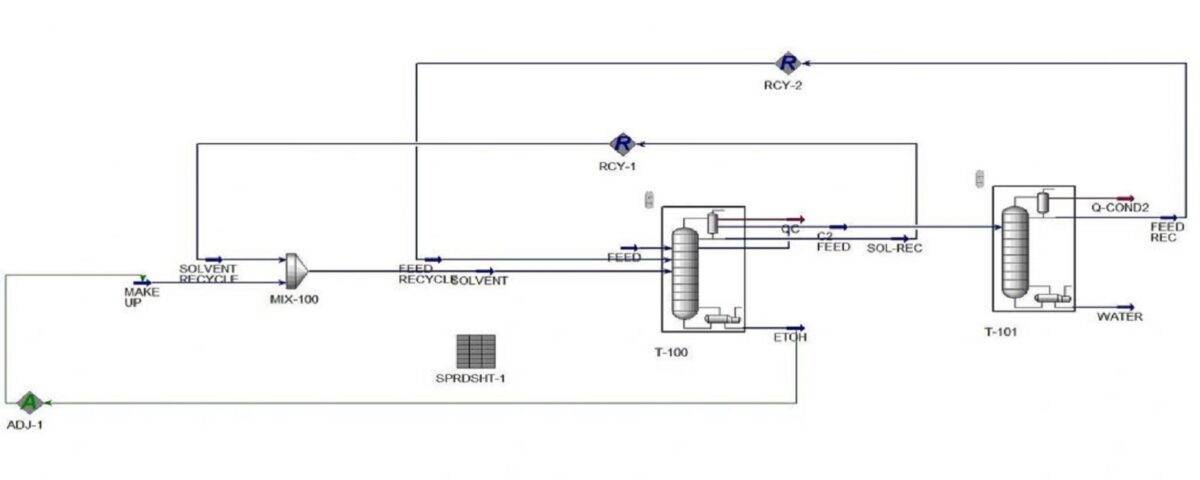
An example of azeotropic distillation is the use of cyclohexane to completely separate ethanol from water. Cyclohexane forms a low-boiling azeotrope with alcohol. A water-alcohol mixture containing 95% by weight alcohol is fed into the azeotropic distillation column, and a benzene-rich stream is fed in from the top. The bottom product is nearly pure alcohol, and the overhead vapor is a ternary azeotrope. This vapor is condensed and separates into two phases. All cyclohexane and a portion of the alcohol in the overhead vapor are returned to the first column. The bottom stream in the third column is distilled to obtain pure water and some binary azeotrope.

Simulation of Azeotropic Distillation Tower for Water and Ethanol Separation
The separation of binary or multicomponent mixtures is a common challenge in the chemical industry. Distillation is often employed for this purpose, leveraging the differences in boiling points of the mixture’s components. However, some mixtures form azeotropes, which exhibit non-ideal behavior and cannot be separated by simple distillation. This means that the composition of the vapor produced from the mixture is identical to the remaining liquid, and the composition of the mixture does not change with continued distillation.
A well-known example of an azeotrope is the water-ethanol mixture. At a specific ratio, these two substances form an azeotrope with a minimum boiling point. In other words, a mixture of water and ethanol at this ratio has a lower boiling point than either of its pure components. This characteristic makes the complete separation of water and ethanol through simple distillation impossible. In this project, the separation process of water and ethanol with the help of cyclohexane in an azeotropic distillation tower has been simulated with Aspen Hysys software.