Description
Batch distillation is one of the common methods for separating mixtures, but the separation of azeotropic mixtures is not possible by usual methods. For this purpose, different methods should be used, such as adding intermediate materials, which changes the thermodynamic properties and changes the position of the azeotrope points or their removal.
Azeotropic Distillation
This distillation method is usually used in cases where the boiling point of the mixture components are close together, it is possible to separate the initial mixture by increasing a special solvent that forms an azeotope with one of the key components. The azeotrope forms the distillate or residue from the column and then separates the solvent and the key component. Often, the added substance forms an azeotrope with a low boiling point, which is called a fragile azeotrope. Azeotropes often contain feed components, but the ratio of key components to other feed components is very different and higher.
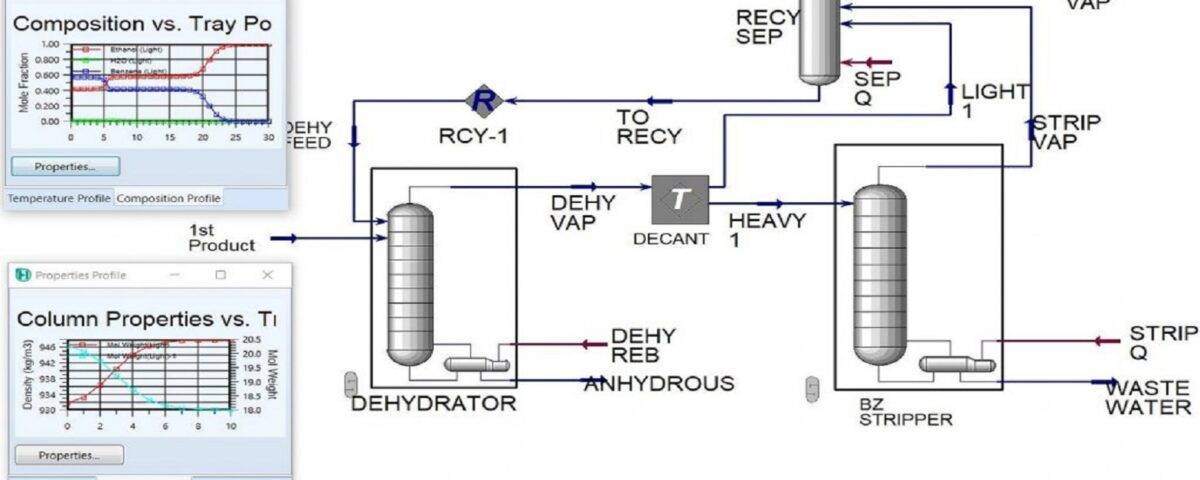
An example of azeotropic distillation is the use of benzene to completely separate ethanol from water, which forms a low-boiling azeotrope with 95.6 wt% alcohol. A water-alcohol mixture with 95 wt% alcohol is added to the azeotropic distillation column and the benzene-rich stream is introduced from the top. The alcohol residue is almost pure and the upper vapor is a triple azeotrope. This liquefied steam is divided into two phases. The returned organic layer is sent to the benzene recovery column. All the benzene and the amount of alcohol captured in the upper steam are sent to the first column. The final stream is distilled in the third column to obtain pure water and some double azeotrope.

Simulation of Azeotropic Distillation Tower for Water and Eethanol Separation
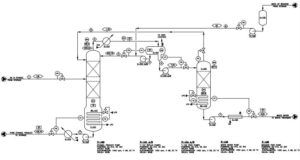
In this project, methods of separation of multi-component mixtures that form azeotropes have been investigated. These methods include the use of two distillation towers at different pressures and the use of an extractant to achieve a purity higher than the azeotrope point. The first method is used for mixtures whose azeotrope point composition is sensitive to pressure. And if the composition of the azeotrope point does not change significantly with the pressure, the second method is used. The studied system is ethanol, water and benzene. Ethanol and water form an azeotrope in the composition of 96% by weight of ethanol, and benzene is used as a remover to separate ethanol up to 99.99%. In order to simulate this separation unit, Aspen Hysys software has been used in steady state.
The following PDF shows the process by which the simulation was carried out.
In addition to the simulation files, this project also has an educational video.