Description
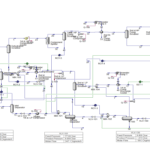
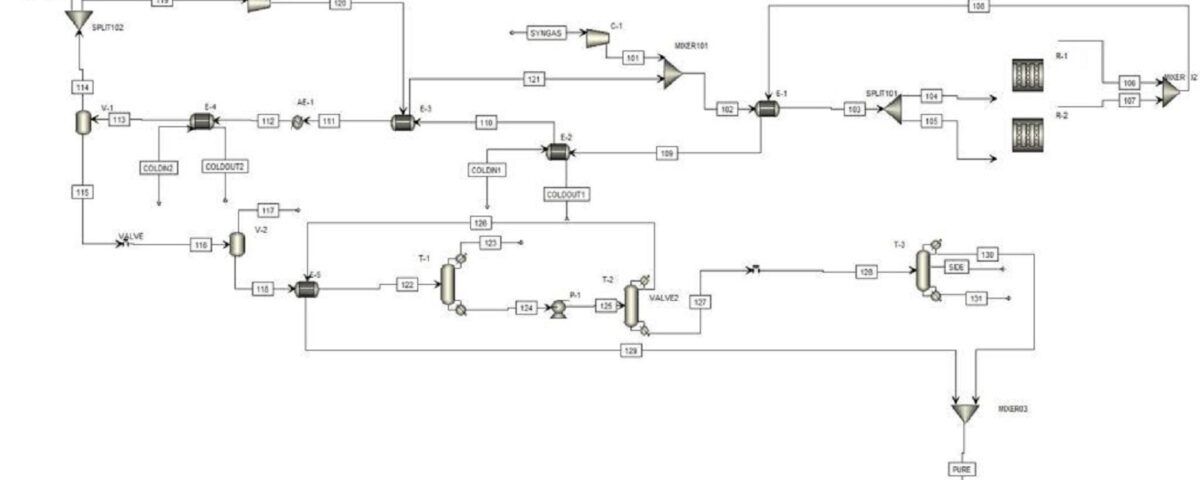
The SHIRAZ Pet. Co. Methanol Unit, with a nominal capacity of 250 tons per day, is recognized as one of the primary methanol production facilities in the country. The aim of this article is to evaluate and analyze the kinetic equations of methanol production based on CO2 consumption and to compare the obtained results with the data of an experimental unit. In this unit, the incoming natural gas is first converted into synthesis gas and finally into methanol. The primary feedstock for the methanol production unit is natural gas and carbon dioxide.
This feedstock, after desulfurization, enters the reformer along with steam and is converted into synthesis gas containing hydrogen, carbon monoxide, and carbon dioxide at a temperature of approximately 900 degrees Celsius. The produced synthesis gas is cooled, compressed, and converted into crude methanol in the synthesis section reactors. After separating the existing water, pure methanol is produced in the distillation unit. The basis of the reactions used is considered based on a mixture of methane and ethane according to actual conditions.
Simulation
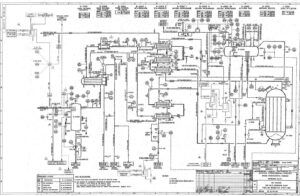
The simulation has been performed in both Aspen Plus and Aspen HYSYS software. The simulation is accompanied by a complete report along with process flow diagrams (PFDs) and piping and instrumentation diagrams (P&IDs) of the Shiraz Methanol Unit, which will be provided to you upon purchasing this product along with the complete work report. A feasibility study (project design and economics) of this unit has also been conducted.
Feasibility Study of Methanol Unit:
Economic feasibility of methanol synthesis as a method for CO2 reduction and energy storage