Introduction
You have recently been assigned to the ethylbenzene (EB) plant at the XYZ petrochemical facility. The center produces a wide range of monomers, polymers and solvents, all of which are derived from petroleum. The EB process produces 80,000 tons/year of 99.8 mol% ethylbenzene, which is completely consumed by the on-site styrene facility. As with most EB/styrene installations, there is significant thermal integration between the two plants. In order to separate the operation of two power plants, the integration of energy is achieved by the production and consumption of steam in two processes. The EB reaction is exothermic, so steam is produced, and the styrene reaction is endothermic, so energy is consumed in the form of steam.
Description of the Process
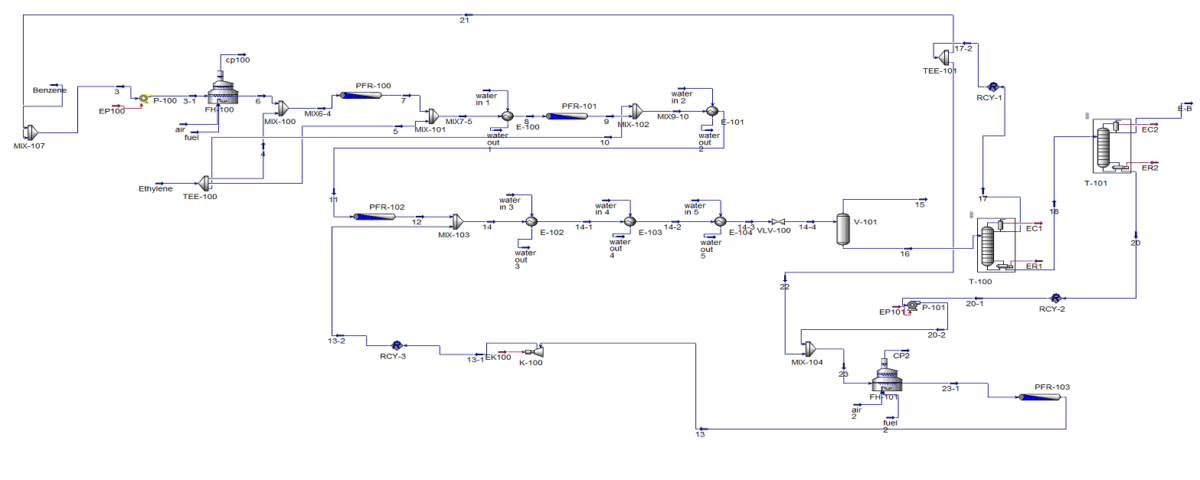
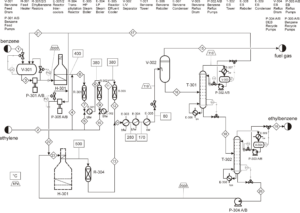
This process produces ethylbenzene (EB). The feed benzene is pumped from the storage tank to the process tank (V-301) and mixed with the recycled benzene. Then this mixture is compressed to a pressure of about 2000 kPa and sent to a heater (H-301) to increase the temperature to about 400 degrees Celsius.
Preheated benzene is mixed with ethylene and enters three fixed bed reactors. The reaction is in gas phase and exothermic. The output of each reactor is mixed with ethylene and then cooled.
In this step, high pressure steam is produced, which is used in the styrene unit. After leaving the third reactor, the output stream does not contain products, impurities, benzene and ethylene. This stream is cooled in two heat recovery boilers where high and low pressure steam is produced.
The two-phase mixture output from the second boiler is sent to a cooler and then two-phase separator. Light gases are used as fuel and the condensed liquid is sent to the benzene tower. Recycled benzene is removed from the top of the tower and returned to the beginning of the process.
The bottom product of the first tower is sent to the second tower, where high-purity ethylbenzene is collected as the top product of the tower. The bottom product of the second tower contains diethylbenzene and small amounts of higher compounds.
This stream is mixed with recycled benzene and sent to the fourth reactor to convert diethylbenzene to ethylbenzene. The output of this reactor is mixed with the input flow to the first boiler.
Conclusion
The production of ethylbenzene from benzene is an important industrial process that is widely used in the chemical and petrochemical industries. Due to the importance of styrene and polystyrene in daily life, the production of ethylbenzene will continue to be a vital part of the chemical industry. However, to maintain environmental sustainability and respond to the growing needs of society, it is necessary to develop new technologies and improve production processes.
Simulation of Ethylbenzene Production Process from Benzene Chain
In this project, the simulation of the production process of ethylbenzene from the benzene chain has been simulated in Span Hysys version 14 software. The project has a complete report.